Académique Documents
Professionnel Documents
Culture Documents
Temperature and Heat
Transféré par
JM LomoljoDescription originale:
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Temperature and Heat
Transféré par
JM LomoljoDroits d'auteur :
Formats disponibles
Temperature Conversion
Thermal Expansion
THERMODYNAMICS (HEAT OF FUSION)
SPECIFIC HEAT 31. What will be the final temperature of a system consisting of a 150-g aluminum cup containing 820 g of water at 12.0 and a 270-g block of copper at 300C?
31.
symbol TCu Ti mCu mAl mw CCu CAl Cw Tf
Givens and Constants quantity initial temperature of copper initial temperature of aluminum and water mass of copper mass of aluminum mass of water specific heat of copper specific heat of aluminum specific heat of water equilibrium temperature
value 300C 12.0C 270 g 150 g 820 g 0.38 J/gK 0.92 J/gK 4.186 J/gK ? C
The heat lost by the copper is the heat gained by the aluminum and water if the heat loss to the surroundings is negligible.
-CCumCu(Tf - TCu) = CAlmAl(Tf - Ti) + Cwmw(Tf - Ti)
-(0.38)(270)(Tf - 300 ) = (0.92)(150)(Tf - 12 ) + (4.186)(820)(Tf - 12 ) Tf = 21.7 C
Temperature and thermal expansion
11-29-99 Sections 13.1 - 13.6 We'll shift gears in the course now, moving from the physics of mechanical systems to thermal physics.
Temperature scales
In the USA, the Fahrenheit temperature scale is used. Most of the rest of the world uses Celsius, and in science it is often most convenient to use the Kelvin scale. The Celsius scale is based on the temperatures at which water freezes and boils. 0C is the freezing point of water, and 100 C is the boiling point. Room temperature is about 20 C, a hot summer day might be 40 C, and a cold winter day would be around -20 C. To convert between Fahrenheit and Celsius, use these equations:
The two scales agree when the temperature is -40. A change by 1.0 C is a change by 1.8 F. The Kelvin scale has the same increments as the Celsius scale (100 degrees between the freezing and boiling points of water), but the zero is in a different place. The two scales are simply offset by 273.15 degrees. The zero of the Kelvin scale is absolute zero, which is the lowest possible temperature that a substance can be cooled to. Several physics formulas involving temperature only make sense when an absolute temperature (a temperature measured in Kelvin) is used, so the fact that the Kelvin scale is an absolute scale makes it very convenient to apply to scientific work.
Measuring temperature
A device used to measure temperature is called a thermometer, and all thermometers exploit the fact that properties of a material depend on temperature. The pressure in a sealed bulb depends on temperature; the volume occupied by a liquid depends on temperature; the voltage generated across a junction of two different metals depends on temperature, and all these effects can be used in thermometers.
Linear thermal expansion
The length of an object is one of the more obvious things that depends on temperature. When something is heated or cooled, its length changes by an amount proportional to the original length and the change in temperature:
The coefficient of linear expansion depends only on the material an object is made from. If an object is heated or cooled and it is not free to expand or contract (it's tied down at both ends, in other words), the thermal stresses can be large enough to damage the object, or to damage whatever the object is constrained by. This is why bridges have expansion joints in them (check this out where the BU bridge meets Comm. Ave.). Even sidewalks are built accounting for thermal expansion. Holes expand and contract the same way as the material around them.
Example
This is similar to problem 12.20 in the text. Consider a 2 m long brass rod and a 1 m long aluminum rod. When the temperature is 22 C, there is a gap of 1.0 x 10-3 m separating their ends. No expansion is possible at the other end of either rod. At what temperature will the two bars touch?
The change in temperature is the same for both, the original length is known for both, and the coefficients of linear expansion can be found from Table 12.2 in the textbook.
Both rods will expand when heated. They will touch when the sum of the two length changes equals the initial width of the gap. Therefore:
So, the temperature change is:
If the original temperature was 22 C, the final temperature is 38.4 C.
Thermal expansion : expanding holes
Consider a donut, a flat, two-dimensional donut, just to make things a little easier. The donut has a hole, with radius r, and an outer radius R. It has a width w which is simply w = R - r. What happens when the donut is heated? It expands, but what happens to the hole? Does it get larger or smaller? If you apply the thermal expansion equation to all three lengths in this problem, do you get consistent results? The three lengths would change as follows:
The final width should also be equal to the difference between the outer and inner radii. This gives:
This is exactly what we got by applying the linear thermal expansion equation to the width of the donut above. So, with something like a donut, an increase in temperature causes the width to increase, the outer radius to increase, and the inner radius to increase, with all dimensions obeying linear thermal expansion. The hole expands just as if it's made as the same material as the hole.
Volume thermal expansion
When something changes temperature, it shrinks or expands in all three dimensions. In some cases (bridges and sidewalks, for example), it is just a change in one dimension that really matters. In other cases, such as for a mercury or alcohol-filled thermometer, it is the change in volume that is important. With fluid-filled containers, in general, it's how the volume of the fluid changes that's important. Often you can neglect any expansion or contraction of the container itself, because liquids generally have a substantially larger coefficient of thermal expansion than do solids. It's always a good idea to check in a given situation, however, comparing the two coefficients of thermal expansion for the liquid and solid involved. The equation relating the volume change to a change in temperature has the same form as the linear expansion equation, and is given by:
The volume expansion coefficient is three times larger than the linear expansion coefficient. Heat Temperature and Thermal Expansion Exam2 and Problem Solutions 1. If Celsius thermometer shows the temperature of air 300C, find the temperature of air in Fahrenheit thermometer.
T(K)=T(C)+273 T=30+273=3030K C/100=(F-32)/180 30/100=(F-32)/180 F=860F 2. Find heat required to make 5g ice at -200C to water at 300C. (cice=0,5cal/g.0C, Lice=80cal/g, cwater=1cal/g.0C)
Heat required to make ice at -200C to ice at 00C ; Q1=m.cice.T=5.0,5.20 Q1=50cal. Heat required to make it melt; Q2=m.Lice=5.80 Q2=400cal. Heat to make it water at 300C; Q3=m.cwater.T=5.1.30 Q3=150cal Qtotal=Q1+Q2+Q3=50+400+150=600cal
3. Two taps fill the water tank with different flow rates. Tap A fills the tank in 1 hour and tap B fills the tank in 3 hour If we open two taps together, find the final temperature of the water in the tank.
Flow rates of taps; VA=3VB 3m.c.(T-10)=m.c(50-T) T=200C
4. When we decrease the temperatures of the rods T, relation between the final lengths of rods becomes; L1<L2<L3. Find the relation between A, B and C.
Since change in length of the rod 1 is larger than the rod 2; A<B Since change in length of the rod 2 is larger than the rod 3; C<A C<A<B
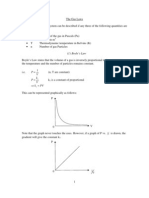
5. Length vs. temperature graph of A, B and C is given below. Find the relation between A, B and C.
Slope of the graph=L/T=L0. Initial length of A is larger than C but slopes of them are equal, so; C>A B and C have same length but slopes of them are different. B >C B >C>A
Vous aimerez peut-être aussi
- SFG 3023 Chapter 1Document67 pagesSFG 3023 Chapter 1Nik AshrafPas encore d'évaluation
- TemperatureDocument6 pagesTemperatureNur Khairiah Daimah SanupinPas encore d'évaluation
- Chapter 3.Document15 pagesChapter 3.Hannah Bee SordillaPas encore d'évaluation
- P103 Chapter10 TAT wk3wk4Document62 pagesP103 Chapter10 TAT wk3wk4Muhammad SaeedPas encore d'évaluation
- G10 CH 6 Heat&Temperature11May07Document17 pagesG10 CH 6 Heat&Temperature11May07Tun Lin AungPas encore d'évaluation
- Bansal CLasses Physics Study Material For IIT JEuEDocument630 pagesBansal CLasses Physics Study Material For IIT JEuESuman KunduPas encore d'évaluation
- PHYSICS 1E Module 9 (Edited)Document54 pagesPHYSICS 1E Module 9 (Edited)Claire G. MagluyanPas encore d'évaluation
- Temperature & Heat (Ok)Document27 pagesTemperature & Heat (Ok)Marhayati Setia NingrumPas encore d'évaluation
- CH 12Document91 pagesCH 12Saranya HarikrishnanPas encore d'évaluation
- ThermodynamicsDocument14 pagesThermodynamicsCarl Angelo MartinPas encore d'évaluation
- Thermal PhysicsDocument24 pagesThermal PhysicsSuraj GopaulPas encore d'évaluation
- Fluid and Thermal ExitDocument25 pagesFluid and Thermal Exitdavididosa40Pas encore d'évaluation
- CHPT 11Document9 pagesCHPT 11MayankPas encore d'évaluation
- Thermal Physics IB Physics: Topic 3 & Option CDocument55 pagesThermal Physics IB Physics: Topic 3 & Option CMoiz Dhanerawala100% (1)
- Bansal CLasses Physics Study Material For IIT JEEDocument521 pagesBansal CLasses Physics Study Material For IIT JEEmuno93% (14)
- Kwame Nkrumah University of Science and Technology, Kumasi Department of Physics Formal Lab ReportDocument20 pagesKwame Nkrumah University of Science and Technology, Kumasi Department of Physics Formal Lab ReportBustenero Dhywill Yaw DraviePas encore d'évaluation
- Heat and Thermodynamics-SubjectiveDocument8 pagesHeat and Thermodynamics-SubjectiveNeeraj KalraPas encore d'évaluation
- 06082021024459am - S.1 Physics Heat IiDocument41 pages06082021024459am - S.1 Physics Heat Iimarionmbambu9Pas encore d'évaluation
- Kuliah TemperaturDocument19 pagesKuliah TemperaturDedy Setiawan ستياوانPas encore d'évaluation
- 17 Thermal-Heat and Kinetics GasDocument34 pages17 Thermal-Heat and Kinetics Gaskirana wahyuniPas encore d'évaluation
- Temperature and HeatDocument48 pagesTemperature and Heatalexzandrei.rara937Pas encore d'évaluation
- Ideal Gas-Worked ExamplesDocument4 pagesIdeal Gas-Worked ExamplesMuhammad MosaPas encore d'évaluation
- 01 Thermal Expansion of MatterDocument6 pages01 Thermal Expansion of MatterElay DyPas encore d'évaluation
- Calorimetry ThermodynamicsDocument15 pagesCalorimetry Thermodynamicssanits591Pas encore d'évaluation
- Lesson 7. Heat, Heat Transfer, Linear and Volume ExpansionsDocument40 pagesLesson 7. Heat, Heat Transfer, Linear and Volume Expansionsmark galangPas encore d'évaluation
- L102 13bDocument33 pagesL102 13belangovanvnrPas encore d'évaluation
- Heat and ThermometryDocument8 pagesHeat and ThermometryElizabeth AnyegaPas encore d'évaluation
- Phys 31 Module 4Document42 pagesPhys 31 Module 4Coyzz de GuzmanPas encore d'évaluation
- Lec. 2Document32 pagesLec. 2Ali. AboudPas encore d'évaluation
- Lesson 4.1 (Smtai 09) .Document5 pagesLesson 4.1 (Smtai 09) .Ilman MohamadPas encore d'évaluation
- Chapter Four Heat and ThermodynamicsDocument44 pagesChapter Four Heat and ThermodynamicsmesfinPas encore d'évaluation
- Thermal Physics Slideshow/Presentation For Edexcel A LevelsDocument41 pagesThermal Physics Slideshow/Presentation For Edexcel A LevelsMustafa AsimPas encore d'évaluation
- Thermal Properties of MatterDocument9 pagesThermal Properties of MatterTrillionare HackPas encore d'évaluation
- Medical Physics, Lecture-5.Ppt (Compatibility Mode)Document30 pagesMedical Physics, Lecture-5.Ppt (Compatibility Mode)fadhaliPas encore d'évaluation
- PHY12 Report 1Document7 pagesPHY12 Report 1Paul JavenPas encore d'évaluation
- Thermal PhysicsDocument66 pagesThermal PhysicsguiPas encore d'évaluation
- Sample Computation: Aluminum CopperDocument5 pagesSample Computation: Aluminum CopperJohn MegryanPas encore d'évaluation
- Thermal PhysicsDocument68 pagesThermal PhysicsAbdul HalimPas encore d'évaluation
- Heat Capacity: Textbook of Heat. Copies of The Relevant Sections Are Available atDocument14 pagesHeat Capacity: Textbook of Heat. Copies of The Relevant Sections Are Available atSunday Glo M. CabuyaoPas encore d'évaluation
- PS21 FinalsReviewerDocument2 pagesPS21 FinalsReviewerIan De La CruzPas encore d'évaluation
- Q4-WEEK 2-Charles's LawDocument21 pagesQ4-WEEK 2-Charles's LawAdonis SanielPas encore d'évaluation
- Phy CH 8 Final 9thDocument28 pagesPhy CH 8 Final 9thmastersahb302Pas encore d'évaluation
- Temperature: Heat TransferDocument33 pagesTemperature: Heat TransferDave MendozaPas encore d'évaluation
- 699908fe-9802-4080-ae15-004131ad3c25Document10 pages699908fe-9802-4080-ae15-004131ad3c25Herton FotsingPas encore d'évaluation
- PHYSICS XI CH-11 (Thermal Properties of Matter)Document28 pagesPHYSICS XI CH-11 (Thermal Properties of Matter)Nandita JainPas encore d'évaluation
- Bansal CLasses Physics Notes For IIT JEEDocument816 pagesBansal CLasses Physics Notes For IIT JEEBram44% (9)
- Lecture 5, Week 3 (Thermal Expansion)Document18 pagesLecture 5, Week 3 (Thermal Expansion)Umer MajeedPas encore d'évaluation
- Chapter (1) Temperature and ThermometryDocument4 pagesChapter (1) Temperature and ThermometryBǿ DYPas encore d'évaluation
- LectureFME16Materials Science and Engineering4Document14 pagesLectureFME16Materials Science and Engineering4Cllyan ReyesPas encore d'évaluation
- Heat and TemperatureDocument26 pagesHeat and TemperatureWanMardziyyahPas encore d'évaluation
- Questions On ConceptsDocument3 pagesQuestions On ConceptsNicole LeinesPas encore d'évaluation
- Worked Problems in Heat, Thermodynamics and Kinetic Theory for Physics Students: The Commonwealth and International Library: Physics DivisionD'EverandWorked Problems in Heat, Thermodynamics and Kinetic Theory for Physics Students: The Commonwealth and International Library: Physics DivisionÉvaluation : 4 sur 5 étoiles4/5 (3)
- Climate change - ocean acidity: Matemaattinen analyysiD'EverandClimate change - ocean acidity: Matemaattinen analyysiPas encore d'évaluation
- Syllabus For Acctg101 (Fundamentals of Accounting I) : Philippine Advent College - Salug CampusDocument9 pagesSyllabus For Acctg101 (Fundamentals of Accounting I) : Philippine Advent College - Salug CampusJM LomoljoPas encore d'évaluation
- 44 Degrees: The If I Were An Archer Fish Page From Lesson 1Document6 pages44 Degrees: The If I Were An Archer Fish Page From Lesson 1JM LomoljoPas encore d'évaluation
- VolcanoDocument84 pagesVolcanoJM LomoljoPas encore d'évaluation
- Ap OrdersDocument1 pageAp OrdersJM LomoljoPas encore d'évaluation
- Measurement Reading Thermometers WorksheetDocument2 pagesMeasurement Reading Thermometers Worksheetportmore community collegePas encore d'évaluation
- Specific Heat Capacities WorksheetDocument2 pagesSpecific Heat Capacities WorksheetRebeca RiveraPas encore d'évaluation
- Psychrometry. Heat Recovery Air Handling Unit (Ahu) - by Ömer Faruk DDocument13 pagesPsychrometry. Heat Recovery Air Handling Unit (Ahu) - by Ömer Faruk DChaminda KumaraPas encore d'évaluation
- Temp Humidity Control - FinalDocument97 pagesTemp Humidity Control - FinalAli HabibPas encore d'évaluation
- Energy Curve POGILDocument5 pagesEnergy Curve POGILCody Palmer100% (1)
- Lecture 07 - ThermochemistryDocument9 pagesLecture 07 - ThermochemistryHyeon Chang NoPas encore d'évaluation
- ChemistryFormative (Ch16)Document2 pagesChemistryFormative (Ch16)dark sparkPas encore d'évaluation
- ThermoDocument121 pagesThermoNikhil Batham83% (6)
- Chapter 01 - Refrigeration PrinciplesDocument19 pagesChapter 01 - Refrigeration PrinciplesLaminTunPas encore d'évaluation
- Assignment 1Document8 pagesAssignment 1Oy BenjiePas encore d'évaluation
- Property Measurement-Pvt: Abstract - The Experiment Was Carried Out ToDocument6 pagesProperty Measurement-Pvt: Abstract - The Experiment Was Carried Out ToAYALEYDENPas encore d'évaluation
- Heat and Thermodynamics (PHYS-103)Document9 pagesHeat and Thermodynamics (PHYS-103)Mubeen GillPas encore d'évaluation
- RAC Domestic Refrigerator Test RigDocument11 pagesRAC Domestic Refrigerator Test RigShashi Bhushan Patel67% (3)
- Summative Test Q2 Week 1 2Document4 pagesSummative Test Q2 Week 1 2Jennelyn G. MalaynoPas encore d'évaluation
- Thermodnamics IAT - I SET - 1 KEY - 1Document2 pagesThermodnamics IAT - I SET - 1 KEY - 1MARSHALPas encore d'évaluation
- VAY Packaged Unit Operation Manual (06.09.18)Document21 pagesVAY Packaged Unit Operation Manual (06.09.18)Nebojsa MijovicPas encore d'évaluation
- How It Works?Document2 pagesHow It Works?saimunPas encore d'évaluation
- Chapter 4 Psychomentry, Humidification and DehumidificationDocument14 pagesChapter 4 Psychomentry, Humidification and DehumidificationNurshaqina SufianPas encore d'évaluation
- GATE Thermo Dynamics Paper 2018Document4 pagesGATE Thermo Dynamics Paper 2018PradyotPas encore d'évaluation
- Calculate Gas Compressibility FactorDocument2 pagesCalculate Gas Compressibility FactorJin Hwan JangPas encore d'évaluation
- Thermodynamic Potentials Unit3Document19 pagesThermodynamic Potentials Unit3Anonymous odl3MBPas encore d'évaluation
- Chemistry 2300: Mol and DH NRLNDocument4 pagesChemistry 2300: Mol and DH NRLNNoreen Guiyab TannaganPas encore d'évaluation
- PsychrometeryDocument11 pagesPsychrometerySohan LalPas encore d'évaluation
- Manual S 2e-V1.00 26dec16Document18 pagesManual S 2e-V1.00 26dec16Faquruddin AliPas encore d'évaluation
- Flight Briefing Package: Release #1Document10 pagesFlight Briefing Package: Release #1Matcha MatchuPas encore d'évaluation
- Analysis of Air-Conditioning Processes Question OnlyDocument4 pagesAnalysis of Air-Conditioning Processes Question OnlyQHalimPas encore d'évaluation
- Casanova: GeometryDocument11 pagesCasanova: GeometryCalin AlexandruPas encore d'évaluation
- Determination-Of-Freezing-Point-DepressionDocument8 pagesDetermination-Of-Freezing-Point-DepressionAllan TampusPas encore d'évaluation
- Spare PartDocument3 pagesSpare PartFuadPas encore d'évaluation