Académique Documents
Professionnel Documents
Culture Documents
Diabetes & Metabolic Syndrome: Clinical Research & Reviews
Transféré par
Riza PermitasariDescription originale:
Titre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Diabetes & Metabolic Syndrome: Clinical Research & Reviews
Transféré par
Riza PermitasariDroits d'auteur :
Formats disponibles
Diabetes & Metabolic Syndrome: Clinical Research & Reviews 3 (2009) 192197
Contents lists available at ScienceDirect
Diabetes & Metabolic Syndrome: Clinical Research & Reviews
journal homepage: www.elsevier.com/locate/dsx
Original paper
Oral health in Thai patients with metabolic syndrome
Umawadee Chomkhakhai a,1, Supanee Thanakun b,*, Siribang-on Piboonniyom Khovidhunkit c,2, Weerapan Khovidhunkit d,3, Sroisiri Thaweboon e,4
a
Dental practitioner, Anundamahidol Hospital, Mueng, Lopburee 15000, Thailand Department of Oral Medicine, Faculty of Dentistry, Mahidol University, 6 Yodhi, Rajthewee, Bangkok 10400, Thailand c Department of Hospital Dentistry, Faculty of Dentistry, Mahidol University, 6 Yodhi, Rajthewee, Bangkok 10400, Thailand d Department of Medicine, Faculty of Medicine Chulalongkorn University Bangkok 10330, Thailand e Department of Oral Microbiology, Faculty of Dentistry, Mahidol University, 6 Yodhi, Rajthewee, Bangkok 10400, Thailand
b
A R T I C L E I N F O
A B S T R A C T
Keywords: Oral health Metabolic syndrome Dry mouth Oral microora
Aim: To study the prevalence of oral manifestations, xerostomia, hyposalivation and level of oral microora in a group of Thai patients with metabolic syndrome (MS) and to determine if there is any association between MS and these oral health components. Methods: Data including patients histories, general health, dental and periodontal status, oral mucosal manifestations, xerostomia, hyposalivation and oral microora in 369 patients with MS were collected and statistically analyzed. Results: Ninety-four subjects (25.5%) were men and 275 (74.5%) were women, with age range from 32 to 88 years (mean = 63.9 10.4). Of these, 231 patients (62.6%) were older than 60 years old. Dental caries in at least 1 tooth and periodontitis were found in 184 (49.9%) and 192 (52.0%) patients, respectively. Oral mucosal manifestations were found in 203 patients (55.0%). The most prevalent manifestation was ssured tongue (41.5%), followed by denture stomatitis (9.2%) and depapillated tongue (3.0%). Dry mucosa was depicted in 203 patients (55.0%). Xerostomia was revealed in 157 patients (42.5%) while hyposalivation was detected in 202 patients (54.7%). Twenty four percent of patients had high Candida level. Signicant association was found between Candida level and hyposalivation and also hyposalivation, xerostomia and dry mucosa. Conclusions: Approximately half of the patients with metabolic syndrome presented with dental caries, periodontitis, dry mouth, oral mucosal changes and approximately one fourth had high Candida level. 2009 Diabetes India. Published by Elsevier Ltd. All rights reserved.
1. Introduction Metabolic syndrome (MS) is a group of interrelated components that predispose individual to increased risk for type2 diabetes mellitus (DM) and cardiovascular disease (CVD). The International Diabetes Federation (IDF) has proposed a denition of MS in order to use for clinical practice and epidemiologic studies [1]. Central obesity, determined by waist circumference, is a prerequisite criterion for the diagnosis of the MS. The minimal waist circumference varies from 90 to 102 centimeters (cm) in males and from 80 to 88 cm in females depending on ethnic group [1]. The MS can be diagnosed when central obesity plus any two of the
* Corresponding author. Tel.: +66 2 203 6500; fax: +66 2 203 6504. E-mail addresses: dtstn@mahidol.ac.th (S. Thanakun), dtspb@mahidol.ac.th (S.-o. Khovidhunkit), wkhovid@gmail.com (W. Khovidhunkit), dtstw@mahidol.ac.th (S. Thaweboon). 1 Tel.: +66 1 802 0840; fax: +66 36 427 401. 2 Tel.: +66 2 203 6530; fax: +66 2 203 6530. 3 Tel.: +66 2 256 4101; fax: +66 2 652 5347. 4 Tel.: +66 2 203 6410; fax: +66 2 203 6410.
following four factors are present. These include raised triglycerides (TG) level (!150 mg/dL, or specic treatment for this lipid abnormality), reduced high density lipoprotein cholesterol (HDLC) (<40 mg/dL in males, and <50 mg/dL in females, or specic treatment for this lipid abnormality), or raised blood pressure (BP) (systolic BP ! 130 or diastolic BP ! 85 mmHg, or treatment of previously diagnosed hypertension) and raised fasting plasma glucose (FPG) (!100 mg/dL, or previously diagnosed type2 DM) [1]. In Thailand, the prevalence of MS varies from 15 to 20% among various population groups which is comparable to those in developed countries [24]. Many drugs used in the treatment of MS can affect salivation and may induce oral complications such as glossitis, oral candidiasis, dental caries and some drugs have been reported to induce oral lichenoid reactions [58]. Moreover, high prevalence of candidiasis, denture stomatitis and periodontitis has been reported in patients with DM, a component of MS [913]. Therefore, we aimed to study the prevalence of oral manifestations, xerostomia, hyposalivation and level of oral microora in a group of Thai patients with MS and to determine if there is any association between MS and these oral health components.
1871-4021/$ see front matter 2009 Diabetes India. Published by Elsevier Ltd. All rights reserved. doi:10.1016/j.dsx.2009.08.004
U. Chomkhakhai et al. / Diabetes & Metabolic Syndrome: Clinical Research & Reviews 3 (2009) 192197
193
Awareness, evaluation of oral conditions and coping with these problems together with the MS treatment could benecially lead to the comprehensive treatment for patients with MS. 2. Materials and methods 2.1. Patient selection Three hundred and eighty two patients attended the Endocrine Clinic of the King Chulalongkorn Memorial Hospital were assessed for MS. In this study, the IDF criteria were used for the diagnosis of MS [1]. All participants were fully informed before completing their written consent document. These protocol and consent forms were approved by The Ethics Committee of the Faculty of Medicine, Chulalongkorn University (Reference Number: 388/2005). First, the waist circumference was measured by a measurement tape placed in a horizontal plane, midway between the inferior margin of the ribs and the superior border of the iliac crest. The inclusion criteria for the diagnosis of MS were waist circumference more than 90 cm in male and 80 cm in female. Then levels of triglyceride, HDL-C, FPG and blood pressure were evaluated from medical chart record. Finally, 369 patients were diagnosed with MS and included in this study. The age, sex, medical and personal history including smoking, drinking and denture hygiene procedure were collected. 2.2. Oral examination The number of patients presented with dental caries in at least 1 tooth was investigated. To evaluate the periodontal status, the Community Periodontal Index (CPI score) was used. The six selected teeth, one permanent molar in each quadrant, one upper central incisor and one lower central incisor, were examined for representative evaluation of all teeth in oral cavity. Healthy gingivae, gingivitis or periodontitis were then classied and recorded. Whole oral mucosa was examined. Normal variation or oral mucosal lesions were recorded. Oral mucosal dryness was evaluated and two categories were classied. Normal moist mouth was determined when most of the oral mucosa was covered with a lm of saliva. Dry mouth was diagnosed when no visible coating of saliva was presented over the entire dorsum of the tongue, buccal and labial mucosa. In addition, if a mouth mirror stuck to these tissues or no pooling of saliva in the oor of the mouth, or if only small discrete amount of frothy saliva was manifested, the patients were subjected to have clinical dry mouth. 2.3. Xerostomia and hyposalivation evaluation Questions indicating xerostomia were asked. These questions were as followed: (1) Do you sip liquids to aid in swallowing dry food? (2) Does your mouth feel dry when eating a meal? (3) Do you have difculties swallowing any food? (4) Does the amount of saliva in your mouth seem too little? And (5) does your mouth usually feel dry? Patients who answered yes in at least 1 question were considered to have xerostomia. Additionally, unstimulated salivary ow rate was measured by Modied Schirmer Test (MST) during 8.0011.00 a.m. Prior to the measurement, the patients were requested not to eat, smoke or brush their teeth for at least 2 h. The patients were asked to sit upright and swallow once to clear the secretion in the mouth. Then the edge of the Schirmer test strip was inserted to the oor of the patients mouth either to the right or the left of the lingual frenum, with the patients tongue slightly raised and gently retracted. The length of strip soaked with saliva as indicated by the level of blue dye was read at the third minute. When the reading was less than 5 mm, it was recorded as 5 mm, and when the reading was greater than
35 mm, it was recorded as 35 mm. The patients were considered to have hyposalivation when the measurement was less than 25 mm in 3 min according to the study of Fontana et al. [14]. 2.4. Oral microora evaluation (Modied Dip-Slide technique) [15] To determine the amount of oral microora related to dental caries and oral candidiasis, the levels of Mutans streptococci, Lactobacilli, and Candida species were assessed. Stimulated whole saliva samples from all patients were collected. Briey, each patient chewed one piece of parafn for 1 min and expectorated all saliva into a sterile container. The saliva was then poured over the surface of a 3-compartment dip-slide containing selective media including Mitis-Salivarius Bacitracin agar, Rogosa SL agar and CHROMagar for the enumeration of Mutans streptococci, Lactobacilli, and Candida species, respectively. The excess saliva was removed by blotting the edge with absorbent paper and then the dip-slide was placed into a plastic tube. Each plastic tube containing dip-slide was incubated at 37 8C for 4872 h in a 5% CO2 incubator. The colonies of Mutans streptococci, Lactobacilli and Candida were counted under a stereomicroscope and checked by Gram staining. The density of the growth of each microora was graded and recorded into 4 levels by comparison with a chart provided with the test [15]. 2.5. Statistical analysis Frequency, percent, the Pearson chi-square test, the Fishers exact test, the MannWhitney U test and the Spearman rank correlation were used where appropriate to assess the association and correlation of variables. p-Value less than 0.05 was considered statistically signicant. 3. Results 3.1. Patient characteristics Of 369 patients with MS, 94 subjects (25.5%) were men and 275 (74.5%) were women. The age varied from 32 to 88 years (mean = 63.9 10.4). One hundred and thirty eight patients (37.4%) were younger than 60 years old and 231 patients (62.6%) were older than 60 years old. The majority of the patients in both genders were 6170 years old. In men, waist circumference varied from 90 to 130 cm (mean = 98.7 8.1). In women, waist circumference varied from 80 to 142 cm (mean = 96.5 9.1). General health status, each component of MS and personal history of patients with MS are presented in Table 1. Male patients were more likely to smoke and drink than female. All patients were taking one or more medications for MS. The number of prescribed medications varied from 1 to 10. Most patients were using 45 drugs. The medications often used by the patients were antilipidaemic, antihypertensive, antidiabetic drugs and acetyl salicylic acid (ASA). Elderly patients (>60 years old) had signicantly used medications daily than younger ones (p = 0.001). Along with these, almost all patients (97%) used xerogenic medications (antilipidaemic, antihypertensive drugs and ASA). 3.2. Oral manifestations One hundred and eighty four patients (49.9%) had dental caries in at least 1 tooth. The mean score of dental caries was 1.42 2.2. One hundred and ninety two patients (52.0%) had periodontitis and 203 patients (55.0%) had one or more oral mucosal manifestations. The most common nding was ssured tongue (153 patients, 41.5%). The distributions of oral mucosal manifestations are presented in Table 2. Of these patients with oral mucosal manifestations, 159
194
U. Chomkhakhai et al. / Diabetes & Metabolic Syndrome: Clinical Research & Reviews 3 (2009) 192197
Table 1 Number of patients according to each component of MS and personal habit. Gender Component of metabolic syndrome Raised triglyceride Male Female Total 77 232 309 (83.74%) Reduced HDL-C 77 231 308 (83.47%) Raised BP 87 238 325 (88.08%) Raised FPG 94 275 369 (100%) Personal habit Smoking 11* 5 16 (4.3%) Drinking 15* 3 18 (4.9%)
Raised triglycerides level; !150 mg/dL. Reduced high density lipoprotein cholesterol (HDL-C); <40 mg/dL in males, <50 mg/dL in females. Raised blood pressure (BP); systolic BP ! 130 mmHg or diastolic BP ! 85 mmHg. Raised fasting plasma glucose (FPG); !100 mg/dL. * p = 0.001.
patients (43.1%) had one manifestation, 38 patients (10.3%) had two and 6 patients (1.6%) had three manifestations. In addition, normal moist oral mucosa was detected in 166 patients (45.0%) whereas dry mucosa was depicted in 203 patients (55.0%). No signicant association between oral mucosal manifestations and age, gender, number of prescribed medications, general health status, complaint of mouth dryness, smoking, level of HbA1c or salivary ow rate was found. Similarly, there was no signicant association between periodontitis and smoking, salivary ow rate or level of HbA1c. In this study, there were 176 (47.7%) denture wearers. Of this group, fty-one patients (29.0%) had partial dentures whereas 125 patients (71.0%) had complete dentures. One hundred and sixty patients (90.9%) cleaned their dentures every day. One hundred and thirty seven patients (77.8%) removed their dentures at night and 127 patients (72.2%) practiced both behaviors. Denture stomatitis was the second manifestation found in 34 patients
(9.2%) and signicantly associated with Candida level (p = 0.006) but not with cleansing of denture or denture removal at night (Table 3). Additionally, there was no signicant association between denture stomatitis and the level of HbA1c (p = 0.49). In this examination, eleven patients (3.0%) presented with glossitis and 8 patients (2.2%) presented with oral lichenoid reaction. Nevertheless, signicant association between these two oral mucosal conditions and possible induced medications were not detected. 3.3. Xerostomia and hyposalivation One hundred and fty seven patients (42.5%) complained that they had dry mouth. There was signicant association between female and complaints of xerostomia (p = 0.004). However, no statistically signicant association was detected between xerostomia and number of xerogenic medications (p = 0.71) or level of HbA1c (p = 0.23). The salivary ow rate in the patients with MS varied from 5 to 35 mm/3 min (mean = 22.68 10.27 mm/3 min). Two hundred and two patients (54.7%) had hyposalivation. Signicant association between xerostomia and hyposalivation was found (p = 0.006). In addition, there was signicant association between the questions Does the amount of saliva in your mouth seem too little? or Does your mouth usually feel dry? with hyposalivation (p = 0.001). Interestingly, the statistically signicant association between dryness of oral mucosa and complaints of xerostomia or hyposalivation was found (p = 0.001) (Table 4). Nevertheless, no signicant association between hyposalivation and age, gender, number of xerogenic medications or level of HbA1c was found in this study. 3.4. Oral microora By Modied Dip-Slide technique, saliva could be collected from 354 patients. The numbers of patients according to level of oral microora are presented in Fig. 1. There was statistically signicant
Table 3 Association between denture stomatitis and related factors. Related factors Denture stomatitis Yes n % 17.0 2.3 No n 130 12 % 73.9 6.8 0.516 p-Value
Table 2 Number of patients with oral manifestations and xerostomia. Oral health status Dental caries 1 tooth 2 teeth More than 3 teeth Periodontal status Healthy gingivae Gingivitis Periodontitis Oral mucosal manifestations Fissured tongue Denture stomatitis (from total subjects) Denture stomatitis (from denture wearers) Depapillated tongue Oral lichenoid reaction or oral lichen planus Traumatic ulcer Varicosity Smokers melanosis Frictional keratosis Other melanin pigmentation Pseudomembranous candidiasis Angular cheilitis Geographic tongue Fibroma Median rhomboid glossitis Oral mucosal dryness Normal moist mucosa Dry mucosa Xerostomia Q1 yes Q2 yes Q3 yes Q4 yes Q5 yes Salivary ow rate Normal (!25 mm/3 min) Hyposalivation (<25 mm/3 min) Number 184 68 46 70 % 49.9 18.4 12.5 19.0
11 166 192 203 153 34 34 11 8 8 8 7 5 5 5 2 2 2 1
3.0 45.0 52.0 55.0 41.5 9.2 19.3 3.0 2.2 2.2 2.2 1.9 1.4 1.4 1.4 0.5 0.5 0.5 0.3
166 203 157 91 91 88 101 119
45.0 55.0 42.5 24.7 24.7 23.9 27.4 32.2
Cleansing denture Yes No
30 4
Denture removal at night Yes 23 No 11 Candida level 1 (low) 2 (medium) 3 (high) 4 (very high)
13.0 6.3
114 28
64.8 15.9
0.111
167 202
45.3 54.7
8 5 11 8
4.9 3.0 6.6 4.8
57 34 21 22
43.3 20.5 12.7 13.3
0.006
U. Chomkhakhai et al. / Diabetes & Metabolic Syndrome: Clinical Research & Reviews 3 (2009) 192197 Table 4 Association between dryness of oral mucosa, complaints of xerostomia and hyposalivation. Oral mucosal dryness Xerostomia Yes n Moist Dry 54 103 % 14.6 27.9 No n 112 100 % 30.4 27.1 0.001 p-Value Hyposalivation Yes n 17 185 % 4.6 50.1 No n 149 18 % 40.4 4.9 0.001
195
p-Value
Fig. 1. Number of patients at each level of microora. Mutans streptococci: level 1 = <103 CFU/mL, level 2 = !103 to <105 CFU/mL, level 3 = !105 to <106 CFU/mL, level 4 = !106 CFU/mL. Lactobacilli: level 1 = <103 CFU/mL, level 2 = !103 to <104 CFU/mL, level 3 = !104 to <105 CFU/mL, level 4 = !105 CFU/mL. Candida: level 1 = <102 CFU/mL, level 2 = !102 to <103 CFU/mL, level 3 = !103 to <104 CFU/ mL, level 4 = !104 CFU/mL.
association between Candida level and hyposalivation (p = 0.035). While there was no correlation between dental caries with Mutans streptococci level (r = 0.088) and Lactobacilli level (r = 0.105). 4. Discussion Through our review, there has been no study of oral conditions in patients with metabolic syndrome. According to the IDF criteria for the diagnosis of MS, proper waist circumference upon ethnicity is an important factor [1]. For Thai people, no specic cut-off point of waist circumference has been reported during the investigation period. Therefore, in this study, the cut-off point dened for South Asian and Chinese population (more than 90 cm in male and 80 cm in female) has been used for waist circumference evaluation since these ethnic groups have the most similar nature compared to other ethnic groups. Of this studied group, two third were elderly who were older than 60 years old. Unfortunately, all patients in this study were taking one or more medication due to their abnormality in each component of MS. The signicant association between the number of medications and the increasing age has been found. This result is comparable to the study conducted in
elderly Thai people in that the presence of medical conditions was high in the elderly and the incidence of medication use increased with advancing age [16]. Dental caries had been presented in 49.9% of our patients, however, there were no correlations between dental caries and Mutans streptococci level, Lactobacilli level, hyposalivation or glycaemic control. These results are contrast to the previous studies that reported the correlation of dental caries with salivary Mutans streptococci and Lactobacilli [17]. Syrjala et al. presented an association of high level of Mutans streptococci and Lactobacilli with dental caries, especially when glycemic control was poor [18]. Almstahl et al. showed that the increase of Mutans streptococci and Lactobacilli levels was associated with hyposalivation. This can be explained in part by the fact that different diet type and good oral hygiene maintenance in most patients may affect low levels of microora and dental caries in this study. The presence of periodontitis was found in approximately one half of the subjects. In contrast to the previous studies [19,20], periodontitis was not signicantly associated with poor glycaemic control in the present study even though 253 patients (68.6%) were uncontrolled type2 DM (HbA1c more than 6.5%). Since this study is a cross sectional, there may be a limitation in the collection of the longitudinal data. Further cohort study should be conducted to evaluate the progression of periodontitis and the glycemic levels in these patients. The prevalence of oral mucosal manifestations in this study (55.0%) was in the range reported by other studies conducted in the elderly (12.083.6%) [2128]. The commonly found oral mucosal manifestations which were ssured tongue, denture stomatitis, depapillated tongue, lichen planus, traumatic ulcer and varicosity are similar to oral lesions found in elderly Thai population though the prevalence in the study of Jainkittivong et al. was higher [21]. This may be due to the fact that the subjects in the latter study were patients seeking for treatment from a dental school and most of these patients had chief complaints of having oral mucosal lesions but the subjects in this present study were general patients attending the endocrine clinic and had no complaint of oral mucosal disorders. The most common condition found in this study was ssured tongue. Previous studies showed that the prevalence of ssured tongue was in the range of 19.927.3% [23,2831]. This lesion was the most prevalent condition in the study of Santos et al. [29]. In addition, there was a strong correlation between tongue lesions and increasing age [28]. In the study of Guggenheimer et al., ssured tongue was signicantly related to the older subjects who had a longer duration of DM [31]. A manifestation of aging, changing in the salivary ow rate or DM might be the explanation of the pathogenesis of this tongue condition. Prevalence of denture stomatitis in this study (9.2%) was higher than those of generalized elderly population in other studies (1 6%) [22,24,25]. This is not surprising since there were reports about the association between denture stomatitis and type2 DM [10] and almost two third of our patients (68.6%) had uncontrolled type2 DM. A positive relationship between Candida level and the presence of denture stomatitis was also found. This nding is in consistent with another study in that the density of oral Candida in
196
U. Chomkhakhai et al. / Diabetes & Metabolic Syndrome: Clinical Research & Reviews 3 (2009) 192197
denture wearers with denture stomatitis was statistically signicantly higher than non-denture wearers and denture wearers without denture stomatitis [32]. Regarding the third most common oral mucosal manifestation, it was depapillated tongue and 3% of all subjects had this lesion. This result is comparable with several previous studies in elderly (14.4%) [22,23,25,26,28] but less than that of the elderly Thai population (8.2%) investigated in a Thai dental school [21]. The prevalence of oral lichenoid reaction among the elderly was 0.43% in the previous studies [2124,27]. The prevalence of this lesion in our study was in this range (2.2%). Surprisingly, no signicant association between medications and depapillated tongue or oral lichenoid reaction was found in this study although the side effects of medications used have been reported to induce oral lichenoid reaction or depapillated tongue [5]. Indeed, these two lesions have not necessarily occurred in all patients who take these medications. Furthermore, in depapillated tongue, several other causes can induce this lesion including chronic trauma, nutritional deciency, hematological abnormalities, peripheral vascular disease and chronic candidiasis [33]. To evaluate the complaint of dry mouth, questions for determining xerostomia in this study was selected from questionnaires reported to be used effectively in the evaluation of salivary gland hyposalivation [34,35]. The prevalence of xerostomia (42.5%) was higher than those of elderly populations in the previous studies (1239%) [3540]. This might be explained by the fact that almost all subjects in this study (97%) were taking xerogenic medications that can induce the reduction of salivary ow rate [41]. However, the association between the number of xerogenic medications, age and xerostomia has not been found. In addition, there was no association between xerostomia and glycemic control status though all subjects in this study were type2 DM. This result is similar to the study by Chavez et al. which revealed that xerostomia was not associated with poorly controlled diabetes [42]. To determine decreased salivary ow rate from side effects of drugs which were used for treatment of MS, Modied Schirmer test was selected. It is a simple method for unstimulated whole saliva ow rate measurement. Cut-off point value for the diagnosis hyposalivation is varied [43]. Fontana et al. found that saliva secretion between 1 and 25 mm/3 min was a good indication of hyposalivation [14]. Comparable to Zunt et al., they suggested that a cut-off point of 25 mm/3 min may be used to identify patients with hyposalivation and a value of 10 mm/3 min or less might be able to identify patients with severe hyposalivation [44]. The mean unstimulated salivary ow rate of the subjects in this study (22.68 mm/3 min) is closest to that of patients who have certain pathologies (SLE, scleroderma, rheumatoid arthritis or pharmacologically related xerostomia), lower than healthy subjects but higher than patients with Sjogrens syndrome in the study of LopezJornet et al. [45]. It may be assumed that the patients with treatment of MS have decreased salivary ow rate comparable to patients who have some autoimmune diseases or taking medication. There was no association between hyposalivation and number of xerogenic medications in this study. Navazesh et al. reported the effect of duration of prescribed medication to hyposalivation [46]. Consequently, duration of taking medication might inuence this result. Association between xerostomia and hyposalivation has been analyzed in order to select the questions that can be used in clinical evaluation. The present study used four questions of Fox and one question of Narhi [34,35]. These questions have been reported to be associated with hyposalivation, both unstimulated and stimulated whole saliva [34,35,47,48]. Similarly, statistically signicant association between xerostomia and hyposalivation has been found in this study. When each question was analyzed
separately, there was statistically signicant association between the question Does the amount of saliva in your mouth seem too little? or the question Does your mouth usually feel dry? and hyposalivation. This result suggested that both questions may be used clinically to screen for any patients with hyposalivation. The patients who have positive answer should be aware and considered to have decreased salivary ow rate. Besides the use of questions to evaluate hyposalivation, clinical assessment could predict salivary gland function by inspection of oral dryness. Oral mucosal dryness has signicant association with hyposalivation in this study. Similarly, Longman et al. reported that the clinicians subjective assessment of oral dryness was indicative of reduction of unstimulated salivary ow rate [49]. Future study would be performed from the patients with untreated MS in general population. This future information may lead to the best understanding and treatment planning for the people who have MS. Acknowledgements This study was supported by Chulalongkorn University Research Grant 2005. References
[1] idf.org [homepage on the Internet]. The IDF consensus worldwide denition of the metabolic syndrome. Brussels: International Diabetes Federation, available from: http://www.idf.org/ [updated 2006 January 23; cited 2006 March 13]. [2] Pongchaiyakul C, Nguyen TV, Wanothayaroj E, Karusan N, Klungboonkrong V. Prevalence of metabolic syndrome and its relationship to weight in the Thai population. J Med Assoc Thai 2007;90(3):45967. [3] Boonyavarakul A, Choosaeng C, Supasyndh O, Panichkul S. Prevalence of the metabolic syndrome and its association factors between percentage body fat and body mass index in rural Thai population age 35 years and older. J Med Assoc Thai 2005;88(3):12130. [4] Cameron AJ, Shaw JE, Zimmet PZ. The metabolic syndrome: prevalence in worldwide populations. Endocrin Metab Clin N Am 2004;33:35175. [5] Abdollahi M, Radfar M. A review of drug-induced oral reaction. J Contemp Dent Pract 2002;3:119. [6] Tack DA, Rogers RS. Oral drug reactions. Dermatol Ther 2002;15:23650. [7] Scully C. Drug effects on salivary glands: dry mouth. Oral Dis 2003;9:16576. [8] Porter SR, Scully C. Adverse drug reactions in the mouth. Clin Dermatol 2001;18:52532. [9] Willis AM, Coulter WA, Fulton CR, Hayes JR, Bell PM, Lamey PJ. Oral candidal carriage and infection in insulin-treated diabetic patients. Diabet Med 1999;16:6759. [10] Dorocka-Bobkowska B, Budtz-Jorgensen E, Wloch S. Non-insulin-dependent diabetes mellitus as a risk factor for denture stomatitis. J Oral Pathol Med 1996;25:4115. [11] Belazi M, Velegraki A, Fleva A, Gidarakou I, Papanaum L, Baka D, et al. Candidal overgrowth in diabetic patients: potential predisposing factors. Mycoses 2005;48:1926. [12] Ueto E, Osaki T, Yoneda K, Yamamoto T. Prevalence of diabetes mellitus in odontogenic infections and oral candidiasis: an analysis of neutrophil suppression. J Oral Pathol Med 1993;22:16874. [13] Guggenheimer J, Moore PA, Rossie K, Myers D, Mongelluzzo MB, Block HM, et al. Insulin-dependent diabetes mellitus and oral soft tissue pathologies II. Prevalence and characteristics of Candida and candidal lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000;89:5706. [14] Fontana M, Zunt S, Eckert GJ, Zero D. A screening test for unstimulated salivary ow measurement. Oper Dent 2005;30(1):38. [15] Thaweboon B, Thaweboon S, Sopavanit C, Kasetsuwan R. A modied dip-slide test for microbiological risk in caries assessment. Southeast Asian J Trop Med Public Health 2006;37:4004. [16] Jainkittivong A, Aneksuk V, Langlais RP. Medical health and medication use in elderly dental patients. J Contemp Dent Pract 2004;5:3141. [17] Narhi TO, Vehkalahti MM, Siukosaari P, Ainamo A. Salivary ndings, daily medication and root caries in the old elderly. Caries Res 1998;32:59. [18] Syrjala AMH, Niskanen MC, Ylostalo P, Knuuttila MLE. Metabolic control as a modier of the association between salivary factors and dental caries among diabetic patients. Caries Res 2003;37:1427. [19] Novak MJ, Potter RM, Blodgett J, Ebersole JL. Periodontal disease in Hispanic Americans with type 2 diabetes. J Periodontol 2008;79(4):62936. [20] Jansson H, Lindholm E, Lindh C, Groop L, Bratthall G. Type 2 diabetes and risk for periodontal disease: a role for dental health awareness. J Clin Periodontol 2006;33:40814. [21] Jainkittivong A, Aneksuk V, Langlais RP. Oral mucosal conditions in elderly dental patients. Oral Dis 2002;8:21823.
U. Chomkhakhai et al. / Diabetes & Metabolic Syndrome: Clinical Research & Reviews 3 (2009) 192197 [22] Corbet EF, Holmgren CJ, Philipsen HP. Oral mucosal lesions in 6574-yearold Hong Kong Chinese. Community Dent Oral Epidemiol 1994;22: 3925. [23] Lin HC, Corbet EF, Lo ECM. Oral mucosal lesions in adult Chinese. J Dent Res 2001;80(5):148690. [24] Pearson N, Croucher R, Marcenes W, Farrell OM. Prevalence of oral lesions among a sample of Bangladeshi medical users aged 40 years and over living in Tower Hamlets, UK. Int Dent J 2001;51:304. [25] MacEntee MI, Glick N, Stolar E. Age, gender, dentures and oral mucosal disorders. Oral Dis 1998;4:326. [26] Jorge Junior J, de Almeida OP, Bozzo L, Scully C, Graner E. Oral mucosal health and disease in institutionalized elderly in Brazil. Community Dent Oral Epidemiol 1991;19:1735. [27] Vigild M. Oral mucosal lesions among institutionalized elderly in Denmark. Community Dent Oral Epidemiol 1987;15:30913. [28] Avcu N, Kanli A. The prevalence of tongue lesions in 5150 Turkish dental outpatients. Oral Dis 2003;9:18895. [29] Santos PJB, Bessa CFN, Aguiar MCF, do Carmo MAV. Cross-sectional study of oral mucosal conditions among a central Amazonian Indian community, Brazil. J Oral Pathol Med 2004;33:712. [30] Kovac-Kavcic M, Skaleric U. The prevalence of oral mucosal lesions in a population in Ljubljana, Slovenia. J Oral Pathol Med 2000;29:3315. [31] Guggenheimer J, Moore PA, Rossie K, Myers D, Mongelluzzo MB, Block HM, et al. Insulin-dependent diabetes mellitus and oral soft tissue pathologies I. Prevalence and characteristics of non candidal lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000;89:5639. [32] Pipatanagovit P, Itharatana K, Aneksuk V. The prevalence and intra oral distribution of Candida in denture stomatitis. J Dent Assoc Thai 1995;45(3): 10824. [33] Brightman VJ. Diseases of the tongue. In: Lynch MA, Brightman VJ, Greenberg MS, editors. Burkets oral medicine. 9th ed., Philadelphia: J.B. Lippincott; 1994. p. 2647. [34] Fox PC, Busch KA, Baum BJ. Subjective reports of xerostomia and objective measures of salivary gland performance. J Am Dent Assoc 1987;115:5814. [35] Narhi TO. Prevalence of subjective feeling of dry mouth in the elderly. J Dent Res 1994;73(1):205.
197
[36] Thomson WM, Chalmers JM, Spencer AJ, Ketabi M. The occurrence of xerostomia and salivary gland hypofunction in a population-based sample of older South Australians. Spec Care Dentist 1999;19:203. [37] Nederfors T, Isaksson R, Mornstad H, Dahlof C. Prevalence of perceived symptoms of dry mouth in an adult Swedish population-relation to age, sex and pharmacotherapy. Community Dent Oral Epidemiol 1997;25:2116. [38] Gilbert GH, Heft MW, Duncan RP. Mouth dryness as reported by older Floridians. Community Dent Oral Epidemiol 1993;21:3907. [39] Osterberg T, Landahl S, Hedegard B. Salivary ow, saliva pH and buffering capacity in 70-year-old men and women. J Oral Rehabil 1984;11:15770. [40] Locker D. Subjective reports of oral dryness in an older adult population. Community Dent Oral Epidemiol 1993;21:1658. [41] Sreebny LM, Schwartz SS. A reference guide to drugs and dry mouth-2nd edition. Gerodontology 1997;14(1):3347. [42] Chavez EM, Taylor GW, Borrell LN, Ship JA. Salivary function and glycaemic control in older persons with diabetes. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000;89:30511. [43] Eisbruch A, Rhodus N, Rosenthal D, Murphy B, Rasch C, Sonis S, et al. How should we measure and report radiotherapy-induced xerostomia? Semin Radiat Oncol 2003;13(3):22634. [44] Zunt SL, Lee L, Woo SB. Identication of salivary gland hypofunction in the management of oral mucosal disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002;94(2):210. [45] Lopez-Jornet P, Camacho-Alonso F, Bermejo-Fenoll A. A simple test for salivary gland hypofunction using Oral Schirmers test. J Oral Pathol Med 2006;35:2448. [46] Navazesh M, Brightman VJ, Pogoda JM. Relationship of medical status, medications, and salivary ow rates in adults of different ages. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1996;81:1726. [47] Sreebny LM, Valdini A, Brook S, Xerostomia. Part I: relationship to other oral symptoms and salivary gland hypofunction. Oral Surg Oral Med Oral Pathol 1988;66:4518. [48] Bergdahl M. Salivary ow and oral complaints in adult dental patients. Community Dent Oral Epidemiol 2000;28:5966. [49] Longman LP, McCracken CFM, Higham SM, Field EA. The clinical assessment of oral dryness is a signicant predictor of salivary gland hypofunction. Oral Dis 2000;6:36670.
Vous aimerez peut-être aussi
- Xerostomia Hyposalivation and Oral Microbiota in Type 2 Diabetic Patients A Preliminary StudyDocument9 pagesXerostomia Hyposalivation and Oral Microbiota in Type 2 Diabetic Patients A Preliminary StudyYunita ManurungPas encore d'évaluation
- DryMouthDentalProfessionals2011 08Document45 pagesDryMouthDentalProfessionals2011 08Nifarea Anlila VesthiPas encore d'évaluation
- Public Health 309Document5 pagesPublic Health 309Blue TechPas encore d'évaluation
- Self-Perception of Periodontal Health and Associated Factors: A Cross-Sectional Population-Based StudyDocument14 pagesSelf-Perception of Periodontal Health and Associated Factors: A Cross-Sectional Population-Based StudyatikramadhaniPas encore d'évaluation
- Dental and Oral Condition in Leprosy Patients From Serra, BrazilDocument8 pagesDental and Oral Condition in Leprosy Patients From Serra, BrazilSudhakar NaiduPas encore d'évaluation
- Jurnal Karies 1Document7 pagesJurnal Karies 1Suci RahmawatiPas encore d'évaluation
- Review Article: Diabetes: Oral Health Related Quality of Life and Oral AlterationsDocument15 pagesReview Article: Diabetes: Oral Health Related Quality of Life and Oral AlterationseveliiiinPas encore d'évaluation
- Cervino Karbo 1Document2 pagesCervino Karbo 1Malya LarasatiPas encore d'évaluation
- Oral Manifestations in Type-2 Diabetes and Related, 2012Document3 pagesOral Manifestations in Type-2 Diabetes and Related, 2012Juliansyah EfrikoPas encore d'évaluation
- Geriatrics: The Prevalence of Oropharyngeal Dysphagia in Acute Geriatric PatientsDocument9 pagesGeriatrics: The Prevalence of Oropharyngeal Dysphagia in Acute Geriatric PatientsEmillya SariPas encore d'évaluation
- Oral Manifestations A Reliable Indicator For Undiagnosed Diabetes Mellitus PatientsDocument6 pagesOral Manifestations A Reliable Indicator For Undiagnosed Diabetes Mellitus PatientsFajar PriandhikaPas encore d'évaluation
- Medoralv22 I5 p586Document9 pagesMedoralv22 I5 p586DivaPas encore d'évaluation
- Halitosis Amongst Students in Tertiary Institutions in Lagos StateDocument6 pagesHalitosis Amongst Students in Tertiary Institutions in Lagos StateChit Tat Thu LayPas encore d'évaluation
- Polak Et Al-2015-Journal of Clinical Periodontology PDFDocument13 pagesPolak Et Al-2015-Journal of Clinical Periodontology PDFChristine HachePas encore d'évaluation
- Prevalence of Caries and Periodontal Disease Among Indonesian Pregnant WomenDocument8 pagesPrevalence of Caries and Periodontal Disease Among Indonesian Pregnant WomenMaharani Dwi AprilianaPas encore d'évaluation
- Evidence Based Practise of Oral MedicineDocument10 pagesEvidence Based Practise of Oral MedicineshrikantPas encore d'évaluation
- Evaluation of The Oral Health of Hemophiliac Patients in CameroonDocument7 pagesEvaluation of The Oral Health of Hemophiliac Patients in CameroonAthenaeum Scientific PublishersPas encore d'évaluation
- The Long Term Effect of A Preventive Programme On Caries Periodontal Disease and Tooth Mortality in Individuals With Down SyndromeDocument13 pagesThe Long Term Effect of A Preventive Programme On Caries Periodontal Disease and Tooth Mortality in Individuals With Down SyndromeSaipul AnwarPas encore d'évaluation
- Periodontal Care PlanDocument18 pagesPeriodontal Care Planapi-643725836Pas encore d'évaluation
- Journal of Clinical Immunology & MicrobiologyDocument14 pagesJournal of Clinical Immunology & MicrobiologyAthenaeum Scientific PublishersPas encore d'évaluation
- 12 Sequel To Renal Transplant An Oral Physician's PerspectiveDocument5 pages12 Sequel To Renal Transplant An Oral Physician's PerspectivevinhannyPas encore d'évaluation
- Psoriasis PPTTDocument39 pagesPsoriasis PPTTlikhithaPas encore d'évaluation
- The Frequency of Dental Caries in Adult Patients With Gastroesophageal Reflux DiseaseDocument4 pagesThe Frequency of Dental Caries in Adult Patients With Gastroesophageal Reflux DiseasefdhfuwhuPas encore d'évaluation
- Journal Pone 0091059Document5 pagesJournal Pone 0091059AgusBhaktiPas encore d'évaluation
- Health Notions, Volume 4 Number 2 (February 2020) Issn 2580-4936Document5 pagesHealth Notions, Volume 4 Number 2 (February 2020) Issn 2580-4936Kamado NezukoPas encore d'évaluation
- Health Notions, Volume 4 Number 2 (February 2020) Issn 2580-4936Document5 pagesHealth Notions, Volume 4 Number 2 (February 2020) Issn 2580-4936Kamado NezukoPas encore d'évaluation
- Halitosis PDFDocument11 pagesHalitosis PDFAnonymous JwLaPuSy4bPas encore d'évaluation
- Oral Health and Salivary Status in Children With Type 1 Diabetes MellitusDocument8 pagesOral Health and Salivary Status in Children With Type 1 Diabetes MellitusfghdhmdkhPas encore d'évaluation
- Bmri2020 1592910 2Document9 pagesBmri2020 1592910 2jhonatan gaspariPas encore d'évaluation
- Harmouche (2021) Knowledge and Management of Halitosis in France and Lebanon: A Questionnaire-Based StudyDocument13 pagesHarmouche (2021) Knowledge and Management of Halitosis in France and Lebanon: A Questionnaire-Based StudyPhuong ThaoPas encore d'évaluation
- Shanaz FinalDocument7 pagesShanaz FinalDimaz ImanPas encore d'évaluation
- Periodontal Status and Risk Factors in Patients WiDocument16 pagesPeriodontal Status and Risk Factors in Patients Wipennyshevlin1Pas encore d'évaluation
- Perception Halitosis of Students in Al Jouf UniversityDocument5 pagesPerception Halitosis of Students in Al Jouf UniversityChit Tat Thu LayPas encore d'évaluation
- 22826Document9 pages22826Ghina Mukti LuthfiaPas encore d'évaluation
- A Color Atlas of Orofacial Health and Disease in Children and Adolescents - ScullyDocument241 pagesA Color Atlas of Orofacial Health and Disease in Children and Adolescents - ScullyHameleo1000100% (3)
- Podj 4Document6 pagesPodj 4cutchaimahPas encore d'évaluation
- 1 PBDocument11 pages1 PBbayuPas encore d'évaluation
- Oral Tori in Chronic Hemodialysis PatientsDocument7 pagesOral Tori in Chronic Hemodialysis PatientsAbdul Rahman AlmishhdanyPas encore d'évaluation
- Vol09 2 3-5str PDFDocument3 pagesVol09 2 3-5str PDFTaufiqurrahman Abdul DjabbarPas encore d'évaluation
- Oral Health in Children With Leukemia: Review ArticleDocument8 pagesOral Health in Children With Leukemia: Review ArticleNindy TweelingenPas encore d'évaluation
- 2016 Halitosis Current Concepts On Etiology Diagnosis and ManagementDocument11 pages2016 Halitosis Current Concepts On Etiology Diagnosis and ManagementDito AnurogoPas encore d'évaluation
- Periodontal Health Status in Patients With Periodontal Disease: A Descriptive Study Among Emirati PopulationDocument7 pagesPeriodontal Health Status in Patients With Periodontal Disease: A Descriptive Study Among Emirati PopulationIJPHSPas encore d'évaluation
- Jos (4) 2013Document9 pagesJos (4) 2013Neamat Hassan Abu-bakrPas encore d'évaluation
- Introduction To The Oral and Maxillofacial Pathology Focus Issue On "Preneoplastic Oral Epithelial Lesions"Document11 pagesIntroduction To The Oral and Maxillofacial Pathology Focus Issue On "Preneoplastic Oral Epithelial Lesions"jesus ulises estrada cantuPas encore d'évaluation
- The Global Burden of Oral Diseases and Risks To orDocument10 pagesThe Global Burden of Oral Diseases and Risks To orViviane GodoiPas encore d'évaluation
- CDH Mar14 3309-Zenthà Fer Pp27-31 3Document5 pagesCDH Mar14 3309-Zenthà Fer Pp27-31 3Stevie FebriliaPas encore d'évaluation
- Oral Health Knowledge and Behavior Among Adults With DiabetesDocument8 pagesOral Health Knowledge and Behavior Among Adults With DiabetesKarina HernandezPas encore d'évaluation
- 1level of PreventionDocument6 pages1level of Preventionblue nPas encore d'évaluation
- Oral Complications in Pediatric Oncology Patients: Stephen A. Fayle, Martin E.J. CurzonDocument7 pagesOral Complications in Pediatric Oncology Patients: Stephen A. Fayle, Martin E.J. CurzonLiana OpriţăPas encore d'évaluation
- Association of Obesity With Periodontitis, Tooth Loss and Oral Hygiene in Non-Smoking AdultsDocument6 pagesAssociation of Obesity With Periodontitis, Tooth Loss and Oral Hygiene in Non-Smoking AdultsNicolas YoungPas encore d'évaluation
- Art:10.1186/s12903 015 0052 4Document8 pagesArt:10.1186/s12903 015 0052 4Laura Mejia VergaraPas encore d'évaluation
- Dentine Hypersensitivity: Developing a Person-centred Approach to Oral HealthD'EverandDentine Hypersensitivity: Developing a Person-centred Approach to Oral HealthPas encore d'évaluation
- Current Treatment of Oral Candidiasis: A Literature ReviewDocument12 pagesCurrent Treatment of Oral Candidiasis: A Literature ReviewgilangPas encore d'évaluation
- Nutrition Awareness and Oral Health Among Dental Patients inDocument11 pagesNutrition Awareness and Oral Health Among Dental Patients intue minhPas encore d'évaluation
- Lack of Reliable Evidence For Oral Submucous Fibrosis TreatmentsDocument2 pagesLack of Reliable Evidence For Oral Submucous Fibrosis TreatmentsashajangamPas encore d'évaluation
- Knowledge, Attitudes, Practices and Status of Oral Health Among Diabetic Patients at Kikuyu HospitalDocument2 pagesKnowledge, Attitudes, Practices and Status of Oral Health Among Diabetic Patients at Kikuyu HospitalNthie UnguPas encore d'évaluation
- Investigation of Volatile Sulfur Compound Level and Halitosis in Patients With Gingivitis and PeriodontitisDocument11 pagesInvestigation of Volatile Sulfur Compound Level and Halitosis in Patients With Gingivitis and PeriodontitisBilly TrầnPas encore d'évaluation
- Physiotherapy For Improving Mouth Opening & Tongue Protrution in Patients With Oral Submucous Fibrosis (OSMF) - Case SeriesDocument9 pagesPhysiotherapy For Improving Mouth Opening & Tongue Protrution in Patients With Oral Submucous Fibrosis (OSMF) - Case SeriesViral ParekhPas encore d'évaluation
- 1 s2.0 S0020653920326678 MainDocument6 pages1 s2.0 S0020653920326678 MainNadya PuspitaPas encore d'évaluation
- Geriatric Dentistry: Caring for Our Aging PopulationD'EverandGeriatric Dentistry: Caring for Our Aging PopulationPas encore d'évaluation
- Oral Management of Patients Undergoing Radiotherapy and ChemotherapyDocument26 pagesOral Management of Patients Undergoing Radiotherapy and Chemotherapymiss0mePas encore d'évaluation
- Saliva and Dental CariesDocument23 pagesSaliva and Dental CariesKhalid MortajaPas encore d'évaluation
- Pathophysiology Salivary GlandsDocument62 pagesPathophysiology Salivary GlandsDwi Rizky LestariPas encore d'évaluation
- The Importance of Oral Health in Palliative Care Patients: Emma RileyDocument5 pagesThe Importance of Oral Health in Palliative Care Patients: Emma RileyShinta Dewi NPas encore d'évaluation
- Diabetes Mellitus and Prosthodontic Care Chanchal Katariya & Dr. SangeethaDocument3 pagesDiabetes Mellitus and Prosthodontic Care Chanchal Katariya & Dr. SangeethaKrupali JainPas encore d'évaluation
- Oralpatho SEQDocument16 pagesOralpatho SEQKhushboo e ZehraPas encore d'évaluation
- PODJ - Imraan and FarzeenDocument6 pagesPODJ - Imraan and Farzeendaniel_siitompulPas encore d'évaluation
- The Functions of Human Saliva - A ReviewDocument12 pagesThe Functions of Human Saliva - A ReviewRaúl Ernesto Quispe CórdovaPas encore d'évaluation
- Sialogogue and Anti Sialogogue PDFDocument20 pagesSialogogue and Anti Sialogogue PDFleslie kalathilPas encore d'évaluation
- Full Denture Prob SolvingDocument9 pagesFull Denture Prob SolvingfsjPas encore d'évaluation
- Salivary GlandsDocument68 pagesSalivary GlandsTatiana Decuseară100% (1)
- Sjogren 3Document12 pagesSjogren 3Marco Antonio García LunaPas encore d'évaluation
- Acute Effects of Radiation InjuryDocument8 pagesAcute Effects of Radiation InjuryVikas VatsPas encore d'évaluation
- Your Complete Guide To DysphagiaDocument36 pagesYour Complete Guide To DysphagiaFabiola NogaPas encore d'évaluation
- Basic Need For GeriatricsDocument38 pagesBasic Need For GeriatricsMuhammad Yatsrib SemmePas encore d'évaluation
- ORE Part 1 August 2017Document20 pagesORE Part 1 August 2017sajna1980Pas encore d'évaluation
- Icd 10Document59 pagesIcd 10Hendra PranotogomoPas encore d'évaluation
- NurBio Activity 12 Analysis of SalivaDocument4 pagesNurBio Activity 12 Analysis of SalivaAlliyah Jhane0% (1)
- Ejpd 2018 2 4Document8 pagesEjpd 2018 2 4TikaZyka16Pas encore d'évaluation
- Xerostomia Lesson Plan BrenkusDocument6 pagesXerostomia Lesson Plan Brenkusapi-508559499Pas encore d'évaluation
- Gerontological Nursing: Fourth EditionDocument22 pagesGerontological Nursing: Fourth EditionTeh DxjPas encore d'évaluation
- Australian Dental Journal - 2019 - Wu - Halitosis Prevalence Risk Factors Sources Measurement and Treatment A ReviewDocument8 pagesAustralian Dental Journal - 2019 - Wu - Halitosis Prevalence Risk Factors Sources Measurement and Treatment A ReviewDita SyifaPas encore d'évaluation
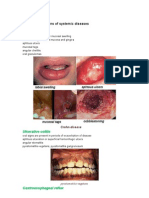
- Oral Manifestations of Systemic DiseasesDocument11 pagesOral Manifestations of Systemic DiseasesMezo Salah100% (2)
- 3rd SeminarDocument75 pages3rd SeminarVeena GoudPas encore d'évaluation
- BATMANGHELIDJ, Dr. F - Your Body's Many Cries For Water (2000) PDFDocument187 pagesBATMANGHELIDJ, Dr. F - Your Body's Many Cries For Water (2000) PDFvperez100% (13)
- Immunity in The Oral CavityDocument1 pageImmunity in The Oral CavityRochman MujayantoPas encore d'évaluation
- Oral Medicine - Update For The Dental Practitioner.: Dry Mouth and Disorders of SalivationDocument5 pagesOral Medicine - Update For The Dental Practitioner.: Dry Mouth and Disorders of SalivationGowriPas encore d'évaluation
- Phtobiomodulation Laser ProtocolsDocument22 pagesPhtobiomodulation Laser ProtocolsIana RusuPas encore d'évaluation
- Symptom Management Guidelines: ORAL MUCOSITIS: Focused Health AssessmentDocument11 pagesSymptom Management Guidelines: ORAL MUCOSITIS: Focused Health AssessmentVincent Paul SantosPas encore d'évaluation
- Salivary Osteocalcin As A Non Invasive Biomarker of Skeletal Maturation - A Cross Sectional StudyDocument4 pagesSalivary Osteocalcin As A Non Invasive Biomarker of Skeletal Maturation - A Cross Sectional StudyInternational Journal of Innovative Science and Research TechnologyPas encore d'évaluation