Académique Documents
Professionnel Documents
Culture Documents
Cushing's Disease / Syndrome - A Knol by Robin S
Transféré par
MaryOCopyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Cushing's Disease / Syndrome - A Knol by Robin S
Transféré par
MaryODroits d'auteur :
Formats disponibles
2/26/12
Cushing's Disease/Syndrome a knol by Robin S
Cushing's Disease/Syndrome
and related endocrine disorders
Cushing's Disease/Syndrome is caused by an overproduction of the hormone cortisol. There are several reasons why that happens. Other endocrine disorders may be caused by or related to the cause for Cushing's. Contents
What is Cushing's Disease/Syndrome? What is cyclic/episodic/intermittent Cushing's? How does diurnal variation/circadian rhythm vary in Cushing's? Testing 101: Introduction Testing 101: Imaging Testing 101: Biochemical Analysis Testing 101: IPSS (aka BIPSS) Testing 101: Growth Hormone Deficiency What types of tumors are associated with Cushing's? more
WhatisCushing'sDisease/Syndrome?
There are two terms used with Cushing's: Disease and Syndrome. They signify the source of the illness, although the presentation is pretty much the same with both. Cushing's Disease is hypercortisolism due to a pituitary source of stimulation. Cushing's Syndrome is hypercortisolism due to an ectopic or adrenal source. There really is a third type of Cushing's called iatrogenic Cushing's which results from the overuse of corticosteriod medications. And actually another type called pseudo-Cushing's. Cushing's Disease/Syndrome (CD/CS) is an endocrine disorder caused by chronic exposure of the body's tissues to excess levels of cortisol - a hormone naturally produced by the adrenal gland. Pituitary adenomas, usually benign, secrete increased amounts of ACTH (adrenocorticotropic hormone), a substance that controls the release of cortisol in that feedback loop I mentioned the other day. It typically causes an overproduction of cortisol. Tumors of the adrenal gland and ectopic ACTH producing tumors can cause similar problems with cortisol overproduction. What are the symptoms? The most common symptoms are: Striae Buffalo hump High blood pressure (often hard to control even with medication)
knol.google.com/k/cushingsdiseasesyndrome# 1/22
2/26/12
Cushing's Disease/Syndrome a knol by Robin S
High blood sugars and/or insulin resistance Insomnia Fatigue Altered diurnal rhythm (See following post) Secondary hypothyroidism Low hormones such as FH, LSH, testosterone, growth hormone Low Vitamin D Low ferritin Unexplained muscle, bone, and joint pain Easy bruising Difficulty when drawing blood Upper body obesity Muscle weakness Increased facial hair/body hair (hirsutism) Loss of hair on head Loss of menstrual cycle and/or ovulation Loss of libido galactorrhea Not everyone has all the symptoms. And the weight gain can vary by individual. Some folks don't gain a lot. Others do.
Without prompt treatment for Cushing's syndrome, other complications may occur, such as: Bone loss (osteoporosis), due to the damaging effects of excess cortisol High blood pressure (hypertension) Kidney stones
knol.google.com/k/cushingsdiseasesyndrome# 2/22
2/26/12
Cushing's Disease/Syndrome a knol by Robin S
Diabetes Unusual infections Hypothyroidism When the cause of Cushing's syndrome is a pituitary tumor (Cushing's disease), it can sometimes lead to other problems, such as interfering with the production of other hormones that the pituitary controls. It can also affect the optic nerves and carotid arteries if large. The majority of pituitary tumors that cause Cushing's disease are small (less than one cm in size). Long-Term Remission Rates After Pituitary Surgery for Cushing's Disease: the Need for Long-Term Surveillance says: Morbidity and mortality are higher in patients with Cushing's disease, with vascular disease a frequent cause of death.[2,32,33] Cardiovascular complications, including coronary heart disease, congestive heart disease and cerebrovascular events, contribute to the morbidity and mortality of patients with undiagnosed or untreated Cushing's disease.[34,35] Early diagnosis and successful treatment of Cushing's disease is therefore most important.
Whatiscyclic/episodic/intermittentCushing's?
Episodic/intermittent/cylic Cushing's has more aliases than a CIA operative. And I'm sure there are some I've left out. Episodic Cushings syndrome (CS) is a rare disorder, characterized by repeated episodes of cortisol excess interspersed by periods of normal cortisol secretion. The so-called cycles of hypercortisolism can occur regularly or irregularly with the phases ranging from days to years. In comparison, "florid" or "classical" Cushing's shows evidence of continual or almost continual hypercortisolism. There is a school of thought which says these are really very rapidly cycling forms of Cushing's. However, there does not seem to be a consensus on that in the literature and in the research. Frankly, I don't know if anyone has done enough testing daily to figure it out. According to some research done in the Netherlands, "As with classic hypercortisolism, cyclic CS is found more commonly among women than men, with a female to male ratio of 3:1 (Table 2). The disorder usually becomes manifest in the fifth decade, but may present from early infancy until older age (highest reported age at presentation being 72 years)." Other clinical studies say, "The features of endogenous hypercortisolism (especially, when mild) are protean and coincide with many common clinical conditions like the dysmetabolic syndrome (1, 2). Screening studies in high-risk populations have discovered unsuspected CS in as many as 25% of patients with diabetes mellitus (37) and suggest that mild CS is more common than previously appreciated." In Cyclical Cushing's syndrome: an update the full text article says, "Cyclical Cushing's syndrome is a pattern of hypercortisolism in which the biochemistry of cortisol production fluctuates rhythmically. This syndrome is often associated with fluctuating symptoms and signs. This type of case was initially thought to be rare. It has, however, recently been recognized as occurring much more frequently. The phenomenon is important because it can, if not recognized, lead to errors in diagnosis and differential diagnosis of the syndrome and in assessment of therapeutic outcomes. All of these can have very serious clinical consequences."
knol.google.com/k/cushingsdiseasesyndrome#
3/22
2/26/12
Cushing's Disease/Syndrome a knol by Robin S
Howdoesdiurnalvariation/circadianrhythmvaryin Cushing's?
Wehearalotaboutcortisolandhowloweringitbyloweringstresscanaffectourweight.I'msure you'veseentheadsforthecortisolslimmingdrugs/herbs,too.Andwearetalkingaboutthe samehormone.Whatiscortisol,anyway? Cortisolisacorticosteroidhormoneproducedbytheadrenalcortex,theouterlayerofthe adrenalglandswhichareessentiallylyingontopofthekidneys.Withoutitwedie.Itisthe hormonethatrespondsto"stress",bothgoodandbad.Itaffectslevelsofmultipleother hormonesandelectrolytes,whichisanothertopicforanotherday.However,theshortofitis,itis veryimportantinthehomeostasisofthebody. Whenthebodyisfunctioningnormally,cortisolisregulatedbythepituitaryinanegative feedbackloopwithACTH(adrenocorticotropichormone).Basically,thepituitaryproduces ACTHwhenthebody'scortisollevelislow,whichstimulatestheadrenalstoproducecortisol. ACTHispulsatile,whichmeansitisproducedinspurtsinsteadofevenlyorconsistently. Ina"normal"person,cortisolisthehighestaround8a.m.anddecreasestoabouthalfthatvalue around4p.m.Bymidnight(giveortakeanhoureachway),cortisolshouldbeaboutzeroor closetoitwithbloodandsalivarylevels.Thisisanormaldiurnalvariation.Itisalsocalledthe circadianrhythm. WhenonehasCushing's,thiscircadianrhythmislost,andthenormaldiurnalvariationchanges. Cushing'spatientshave"flat"diurnallevelsofcortisol,orevenhigherlevelsatnightinsteadofin themorning. Endotext.comexplainsitreallywell.Theirdiagramshowsthe differencebetweennormalserumcortisollevelsandthelevelsfor thosewhohaveCushing's.(Clickonthelinktoseethepicture better.) Wheredoesthattakeus?Well,totesting.Becauseofthischange fromnormaltowarpeddiurnalrhythm(orlackof),testingcortisol levelscanbeveryusefulwhendiagnosingCushing's Syndrome/Disease. Thearticle,"Cushing'sSyndrome"byJohnNewellPrice,XavierBertagna,AshleyBGrossman, LynnetteKNieman,Lancet2006367:160517,DivisionofClinicalSciences,Universityof Sheffield,NorthernGeneralHospital,Sheffield,UK(JNewellPriceFRCP)says: Midnightplasmacortisolorlatenightsalivarycortisol:Normalcircadianrhythmofcortisol secretionislostinpatientswithCushingssyndrome.Asinglesleepingmidnightplasmacortisol concentrationoflessthan50nmol/LeffectivelyexcludesCushingssyndromeatthetimeofthe testandthismightbeespeciallyhelpfulinpatientsinwhomtherehasbeenincomplete suppressionondexamethasonetesting.Concentrationsofmorethan50nmol/Larenotedin individualswithCushingssyndrome,eventhosewhosuppressserumcortisolonlowdose dexamethasonetesting,96butthiscutofflacksspecificitybecausepatientswithacuteillness alsohavevaluesabovethisconcentration.Anawakemidnightconcentrationofcortisolin
knol.google.com/k/cushingsdiseasesyndrome# 4/22
2/26/12
Cushing's Disease/Syndrome a knol by Robin S
plasmaofmorethan207nmol/LdifferentiatesbetweenCushingssyndromeandothercausesof hypercortisolaemiabutcanmissmilddiseasediagnosisinabout7%ofcases.(Theconversion factorofug/dLx27.6=nmol/L)
Testing101:Introduction
Sincedeterminingendocrinedisordersinvolvesalotoftesting,IthoughtIwouldspendsome timetalkingaboutsomeofthosetests.WithCushing'sDisease,anadenomacancause hypopituitarismandpanyhypopituitarismaswellasexcesssecretionofahormoneorhormones fromtheadenomaitself.Inordertounderstandwhattestsaredoneandwhyyouwillfirstneedto knowmoreaboutthepituitaryanddisordersassociatedwithit. Hypopituitarismisadisorderwherethepituitarydoesnotsecreteenoughoranyofoneormore hormones.Theliteraturevariesonthis,however.Somedefineitas"twoormorehormones". However,thebottomlineisthepatientisdeficientandthiswillaffectoneormoreofthebodies functions.Panhypopituitarism,ontheotherhand,isthedeficiencyand/ortotallossofall hormoneproductioninthepituitary. Thepituitaryglandisabeanshaped(thinklima bean)organthatisatthebaseofthebrain.The glandisattachedtothehypothalumus(apartof thebrainthataffectsthepituitarygland)bynerve fibers.Thepituitaryglanditselfconsistsofthree sections:theanteriorlobe,theintermediatelobe, theposteriorlobe.Theintermediatelobeis rudimentaryinhumanbeingsbutproduces severalhormoneswhosephysiologic significanceisonlynowbeingestablished. Eachlobeproducescertainhormones. Anteriorlobe: growth hormone prolactin - to stimulate milk production after giving birth ACTH (adrenocorticotropic hormone) - to stimulate the adrenal glands TSH (thyroid-stimulating hormone) - to stimulate the thyroid gland FSH (follicle-stimulating hormone) - to stimulate the ovaries and testes LH (luteinizing hormone) - to stimulate the ovaries or testes Intermediate lobe: MSH(melanocyte-stimulating hormone)- to control skin pigmentation Posterior lobe:
knol.google.com/k/cushingsdiseasesyndrome# 5/22
2/26/12
Cushing's Disease/Syndrome a knol by Robin S
ADH (antidiuretic hormone) - to increase absorption of water into the bloodby the kidneys oxytocin - to contract the uterus during childbirth and stimulate milk production. It is also believed to be important for orgasm Each of these hormone levels can tell a diagnostician a lot about the state of the pituitary and what may be happening there. The level of each is like a piece of the puzzle, and some need to be measured regularly to get the overall picture. Not only are these hormone levels important, but the levels of other hormones affected by these are measured. In the thyroid, TSH from the pituitary affects the levels of T3 and T4. In the adrenal gland, the level of cortisol is affected by the level of ACTH. It's that feedback loop I'm always talking about. The level of cortisol at various times of the day (8 a.m., 4 p.m., and midnight) is an important tool for diagnosing Cushing's. I've already talked about this some in other articles, and I'll elaborate more in later ones. In post-pubescent females, FSH acts on the ovarian follicles to produce estrogens and LH is instrumental in the production of progesterone. In males, LH is instrumental in the production of testosterone. The hypothalamus secretes gonadotropin-releasing hormone (GnRH) to the pituitary gland in pulses. These, in turn, stimulate the pituitary gland to secrete luteinizing hormone (LH) which then stimulates the Leydig cells of the testes to produce testosterone. That brings us to the hypothalamus. The hypothalamus is a region of the brain above the pituitary. It contains several types of neurons responsible for secreting different hormones. These are released into the blood in the capillaries and travel to the anterior lobe of the pituitary. Corticotropin-releasing hormone (CRH) Thyrotropin-releasing hormone (TRH) Growth hormone-releasing hormone (GHRH) Gonadotropin-releasing hormone (GnRH) Dopamine Somatostatin Each of these plays a role in the production of the hormones in the pituitary. Usually, those pituitary hormones are tested rather than the hypothalamic hormones. The hypothalamic hormones are often used to stimulate the pituitary to see if it is producing those anterior lobe hormones, so they are valuable in the testing process. Altogether, these glands and their hormones comprise the Hypothalamic-Pituitary-Adrenal axis, also known as the HPA-axis.
knol.google.com/k/cushingsdiseasesyndrome#
6/22
2/26/12
Cushing's Disease/Syndrome a knol by Robin S
The adrenal glands sit on top of the kidneys and are sometimes called suprarenal glands. The adrenal gland is actually two glands that are fused together into one gland. Their triangular shape is composed of two main layers as a result: cortex and medulla. The adrenal medulla produces two main chemicals called catecholamines: epinephrine (also called adrenaline) and norepinephrine. Both of these chemicals are involved in regulation of the nervous system. Epinephrine controls the short-term stress response (aka fight-or-flight response) with the help of norepinephrine, which is also involved in the regulation of mood. The outer part of the adrenal gland, also called the adrenal cortex, produces steroid hormones that are involved in regulating a number of different body functions. Mineralocorticoids (such as aldosterone) help regulate the salt levels in the body by controlling the absorption and excretion of salt and water in the kidneys which is important in the regulation of blood pressure. Glucocorticoids (such as cortisol) regulate sugar and fat stores within the body, act as a strong anti-inflammatory force, and play an important role in fetal development, particularly in lung maturation. The adrenal cortex also produces several sex steroid hormones, including androgens (critical for male sexual development) and precursors to estrogen (critical for female sexual development). Tumors of the adrenal glands arise from the cortex or the medulla part of the adrenal gland. Most are found during CT scans or other imaging for various reasons. Others are found due to the effects of the oversecretion of the chemicals or hormones they produce. Steroids: Excess secretion of the steroid hormones produce a Cushings syndrome Aldosterone: Excess secretion of aldosterone produce a Conns syndrome Catecholamines: Excess secretion of catecholamines produce a pheochromocytoma A benign tumor called an adrenal adenoma is the most common and is sometimes called an incidentaloma if it is not causing any hormonal or chemical oversecretions. However, there is a newer line of research which indicates these are not to be taken lightly and may be a result of other endocrine malfunctions. Marc Slawik and Martin Reincke write in Endotext.com: In a study patients with incidentalomas who were suffering from subclinical Cushings syndrome (SCCS) were significantly more obese (17). In addition, patients with incidentalomas more frequently suffer from diabetes mellitus type 2 (2, 5) and it has been postulated that in these individuals hyperinsulinism leads to an increased proliferation of adrenal cells (25). Taking these findings together there seems to be a clear association of incidentalomas with features of the metabolic syndrome (obesity, arterial hypertension, NIDDM, dyslipidemia, dyscoagulation). A recent news article from Harvard Medical School says: The study, lead by Dr. Leslie Eldeiry, a clinical instructor at Harvard Medical School, which was conducted at Harvard Vanguard Medical Associates, found that"only 30 percent of patients underwent biochemical evaluation for adrenal hyperfunction," which is the production
knol.google.com/k/cushingsdiseasesyndrome# 7/22
2/26/12
Cushing's Disease/Syndrome a knol by Robin S
of excessive amounts of hormone. In 2002, the National Institutes of Health released guidelines recommending hormonal evaluation of all incidentally discovered adrenal masses. Despitethe 2002 NIH recommendations, only 30 percent of patients were properly tested. Moreover only 18 percent of patients in the study who did not see an endocrinologist with adrenal nodules had hormonal testing. (New Research Indicates That Adrenal Nodules Not Being Adequately Evaluated ) The most common malignant tumors found in the adrenal gland are tumors that come from cancer cells that have metastasized (or spread) from other parts of the body to the adrenal gland through the blood stream. Rarely, cancers can arise directly within the adrenal glands themselves. Cancers of the adrenal cortex are called adrenal cortical cancers. Functioning adrenal cortical cancers (they secrete excess steroid hormones) are more common than non-functioning cancers. The most common cancer of the medulla are pheochromocytomas. In children, neuroblastoma tumors can develop within the adrenal medulla.
Testing101:Imaging
Since there is so much involved in the testing and diagnosis of Cushing's Syndrome/Disease, I thought I'd insert some information on imaging here. Imaging alone cannot diagnose Cushing's. There must be biochemical proof of it, also, which includes testing those chemicals and hormones I've mentioned. However, negative imaging does not rule out Cushing's, either. A special type of Magnetic Resonance Imaging (MRI) , called a dynamic MRI is the preferred method for pituitary tumors/lesions/adenomas. With a dynamic MRI, a series of MRI images are taken quickly over several minutes after the infusion of gadolinium, a special contrast agent. Dynamic scans are used because pituitary tumors and normal gland tissue absorb the dye at different speeds. The contrast between the normal tissue and tumor is easier to delineate with this type of imaging.Dynamic MRIs may be especially important when a small tumor of the type that causes acromegaly or Cushing's disease is suspected. According to recent research, a dynamic pituitary MRI has high sensitivity and specificity for the diagnosis of mild Cushing's syndrome and should be part of the initial workup. Because the pituitary gland is small, tumors/adenomas/lesions are even smaller and are difficult to spot. Therefore, it is essential the right type of MRI is ordered for you. A pituitary gland MRI is not the same as an MRI of the brain. A brain MRI does not show the pituitary nearly as well. A closed MRI is preferred over an open MRI because the resolution is better. However, since obesity is a symptom of Cushing's, it is sometimes difficult to find a decent MRI big enough. There are some newer "open" MRI's which are 1.5 Tesla and above. (Click here for locations.)Typically, most MRI scanners have a strength of 1.5 Tesla but the newer, 3.0 Tesla scanners are becoming more prevalent and if available, should be used. A computed tomography (CT) scan of the adrenal glands is usually done to localize an adrenal tumor if it has not been found and if a pituitary adenoma has been ruled out. If a discrete adenoma is seen in one adrenal gland and not on the other gland, then that with the biochemical evidence may be enough for a unilateral adrenalectomy. (Just the adrenal gland with tumor is removed.)
knol.google.com/k/cushingsdiseasesyndrome# 8/22
2/26/12
Cushing's Disease/Syndrome a knol by Robin S
If the biochemical evidence is supportive of a pheochromocytoma, again a CT scan of the adrenal glands is performed. Most pheochromocytomas in the adrenal gland are easily visible with a CT scan. If the CT scan does not show one, then there is a chance of an "extra-adrenal" (outside the adrenal gland) pheochromocytomas. The literature supports using one or more of the following methods to find the tumor. iodine-131labeled metaiodobenzylguanidine (131I-MIBG) positron emission tomography (PET) scan octreotide scan (done more in the UK than in the US) abdominal MRI Often, adrenal tumors are found when CT or MRI scans are done for something else. Ectopic tumors are probably the hardest to locate. Often, only after pituitary and adrenal tumors are ruled out are they pursued. Metomidate PET is a highly selective and potentially promising approach of adrenocortical tumours that has been introduced in only few PET centres up to now. A gold standard technique in localizing ectopic ACTH-producing tumors has not been determined, but CT, MRI, and somatostatin receptor scintigraphy (SRS) are all used in various combinations to try to find the tumor(s). This is a bare-bones outline of possible imaging. The research is always telling diagnosticians more, and the biochemical evidence is very, very important in the diagnosis. Imaging is fallible and only as good as the equipment and the person reading the results. It is always good to get multiple opinions from respected surgeons who do a lot of pituitary or adrenal surgeries when reading imaging studies.
WhatisdynamicMRImaging?
Those of us who have suffered or still suffer with pituitary adenomas have heard way too many times "your MRI is normal". I did, for years. Yet, I ultimately did have a proven adenoma which caused my Cushing's disease. If the MRI had shown even an inkling of the tumor to the trained eye, perhaps a doctor would have taken my symptoms more seriously. So, what makes a difference, then? Two things made a huge difference for me. First, my current endocrinologist insisted on a dynamic MRI. Secondly, I sent the films and/or CDs to neurosurgeons who remove a lot of pituitary adenomas. What my local radiologist called a "normal" MR image of my pituitary was actually deemed NOT NORMAL by three worldrenowned neurosurgeons. What is a dynamic MRI? In order to understand that, you need to first understand what an MRI is. Typically, pituitary MRI's are done "without contrast" and "with contrast". The Magnetic Resonance Imaging is done with no radioactivity (aka x-rays). It uses a strong magnetic field produced by a large magnet to send radio waves through the body which "jiggle" the body's atoms. When these atoms move back into place, they send out radio waves of their own which are picked up by the scanner and fed into a computer. This computer then uses programmed algorithms to turn them into pictures. To learn more about it, visit How Stuff Works. A contrast is often used with MR imaging, especially of the head, to enhance the images. Solutions of
knol.google.com/k/cushingsdiseasesyndrome# 9/22
2/26/12
Cushing's Disease/Syndrome a knol by Robin S
gadolinium compounds are typically used as contrast agents. Tumors enhance after gadolinium is given because they tend to absorb the contrast agent either more quickly or less quickly than "normal" tissue. This leads to a "contrast" between the two types of tissue. What makes a dynamic MRI different from any MRI using contrast? Typically, a series of images are taken prior to contrast and then the MR imaging is stopped while contrast is injected. Once that is finished, the MRI proceeds with another series of images. With the dynamic protocol, the contrast is infused over a period of time while the MR imaging is taking place. In one study the gadolinium solution was injected via IV over a period of 180 seconds. In another study the gadolinium was dripped via IV between 2 and 3 minutes. Why does that make a difference? Pituitary tumors and normal gland tissue absorb the gadolinium at different speeds. The contrast between the normal tissue and tumor may be easier to see in the earlier images when compared to the later ones. Usually the pituitary adenoma enhances slower than the gland. (However, there have been documented cases of just the reverse if the tumor encases a blood supply.) When the tumor enhances slower, a "dark spot", in layman terms, shows up on the pituitary. These are called areas of "hypointensity". This is transitory and if not imaged as it happens, the tumor will enhance to match the gland. (In the picture, the upper image does not clearly show a tumor. The lower image shows the tumor well including its contact with the right internal carotid artery.) Although I did not mention the strength of the MRI scanners being used, it's probably obvious that the stronger they are (measured in Tesla), the better they work. A 3T scanner is preferable if available, but the authors of the studies used scanners as low as 0.5T in their studies. A scanner is only as good as those operating it, those reading the scans, and the protocols used not matter how strong it is.
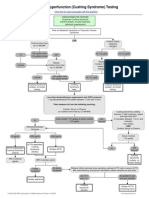
Testing101:BiochemicalAnalysis
I have talked about many of the tests previously. What I want now is outline basic tests that most endocrinologists use for the diagnosis of Cushing's. The following chart is general, and not totally inclusive, but it may help understand why certain tests are run:
knol.google.com/k/cushingsdiseasesyndrome#
10/22
2/26/12
Cushing's Disease/Syndrome a knol by Robin S
This chart is talking about the comparision of serum cortisol and plasma ACTH to known ranges for known times. Serum cortisol should be measured at 8 a.m., 4 p.m., and around midnight (most clinical studies use 11 p.m. - 1 a.m.). If you remember, this will show the diurnal variation (or lack thereof). If the diurnal rhythm is not normal, this is one clue for the diagnosis of Cushing's. According to Esoterix Labs, normal adult ranges for serum cortisol are: 8:00 a.m. 8.0 19 ug/dL 4:00 p.m. 4.0 - 11 ug/dL midnight close to zero ACTH is pulsatile, and should be 9-54 pg/mL during the day. At midnight, however, the clinical studies say it should be less than 23 pg/dL or it is excessive. Refer to "When tests don't even rate an A+ or a C-" to see how to make sure ACTH is tested properly. Often, it's hard to get a valid result. In The Biochemical Investigation of Cushing Syndrome[Neurosurg Focus 16(4), 2004. 2004 American Association of Neurological Surgeons], the last page says: In patients with Cushing disease, 50% have a 9 a.m. plasma ACTH level within the normal reference range of 9 to 54 pg/ml (212 pmol/L) and the remaining patients have a slightly elevated ACTH level.[36] Due to the loss of circadian rhythm, however, nighttime ACTH secretion is abnormal. A midnight plasma ACTH levelgreater than 23 pg/dl (5 pmol/L) confirms the presence of an ACTH excess. Salivary cortisol tests are also done to determine diurnal/circadian rhythm. Since midnight serum cortisols are more difficult to do because the patient has to go to an open lab late at night, salivary kits offer a much easier alternative. However, serum cortisol tests tend to work better for cyclical patients because the serum
knol.google.com/k/cushingsdiseasesyndrome# 11/22
2/26/12
Cushing's Disease/Syndrome a knol by Robin S
level has to be pretty high before the cortisol is readable in saliva. Esoterix has developed a more sensitive assay for testing salivary cortisol which may offer a comparable output. There are four FDA-approved labs for testing salivary cortisol (Quest, ACL Labs, Esoterix, and Labcorp), and each uses it's own method with varying ranges. The ranges for Esoterix are below:
24-hr Urinary Free Cortisol (UFC) is another test that is used. Again, depending on the method used to run these and the lab, the ranges can vary. These are also discussed in "When tests don't even rate an A+ or a C-" . These are used to get an average value of the excess cortisol secreted in a 24-hour period. Some researchers also use a 10-hour UFC to see if excess is just secreted overnight. These are analyzed differently by looking at the cortisol/creatinine ratio. A ratio of 15 or higher is considered diagnostic. There is some question about the validity of the dexamethasone suppression test, with various factions in the literature and in the research saying various things. Basically, it boils down to the dosage used with the dexamethasone and the doctor doing it. An Update on the Overnight Dexamethasone Suppression Test for the Diagnosis of Cushing's Syndrome: Limitations in Patients with Mild and/or Episodic Hypercortisolism [T. C. Friedman, Exp Clin Endocrinol Diabetes. 2006 Jul;114(7):356-60] says: The objective of this study was to determine the sensitivity of the one mg overnight dexamethasone suppression test in patients with mild and/or periodic Cushing's syndrome... Therefore, an overnight dexamethasone suppression test was performed in 17 consecutive patients presenting to an endocrinology clinic with signs and symptoms of hypercortisolemia who were later proven to have Cushing's syndrome... [These patients] failed to suppress to a value less than this cut-off point (sensitivity of 41 %). These results demonstrate that the great majority of patients with mild and/or periodic Cushing's syndrome suppress to overnight dexamethasone. Since patients with mild and/or periodic Cushing's syndrome are the patients in whom the identification of hypercortisolism is difficult, our results from this relatively small study suggest that this test should no longer be
knol.google.com/k/cushingsdiseasesyndrome# 12/22
2/26/12
Cushing's Disease/Syndrome a knol by Robin S
used to exclude these patients from further workup for Cushing's syndrome. Previously I explained the difficulties in diagnosing cyclic/episodic/mild/subclinical Cushing's. Be prepared to do a lot of testing. If you are cyclic, you will have to figure out the symptoms of your "highs" versus your "lows". The only way to do that is to JOURNAL your symptoms and TEST! Keep very detailed records of all lab results. Make sure you get a copy of each. Compare the results to your symptoms. If you are consistent, you can figure out your cycle fairly quickly. In order to do this, you must find a doctor who is willing to let you test when you need to test. Watch out for lab errors: When testing for Cushing's, there are some standard tests that most up-todate, in-the-know endocrine centers/doctors abide by. Of course, when one gets into the current research, combines the complexities of mild/episodic/cyclic Cushing's with florid/classical and then adds a dash ton of no-one-uses-the-same-protocols, no wonder it gets confusing. The Urinary Free Cortisol (UFC) test has long been touted as the "gold standard". The current concensus among those who test episodic (et al) Cushing's the most is that it is NOT, but for the sake of argument and time, let's go with it. Everyone being tested for Cushing's will have to do UFC's. You are a rare one if not. The UFC is a 24-hour collection of urine. That part is pretty easy. You discard the first void, start timing from there and collect every single drop or those 24 hours, ending at the same time you started. What happens to that urine is the big debate, both before and after collection. My endocrinologist and at least two other major endocrinologists who are experts in the field recommend refrigeration if it's not going to the lab immediately (and possibly even then) but NO PRESERVATIVE. Well, getting labs to agree to that is not going to happen. The absolute no-no that I've been told over and over is hydrochloric acid (HCl) as a preservative. Boric acid is accepted by most labs. Acetic acid is mentioned occasionally. In a study done by ARUP Institute, they found "Cortisol concentrations in samples stored with the acids were higher by 30% than in samples stored without acid, possibly as a result of partial hydrolysis of sulfate and glucuronide conjugates. " Esoterix, the diamond of endocrine labs, prefers no preservative at all. What is a UFC measuring anyway? Well, "free" cortisol is used by the body for various functions, but the thought behind the test is that this free cortisol is spilled into the urine if there is excess. So, if there is excess free cortisol spilled into the urine, an average can be measured for a 24-hour period. UFC's are not just used to measure cortisol, though. Most endocrinologists also want to test the amount of creatinine in the urine, and many want to also test 17-hydroxycorticosteriods (17-OHC's). When that is the case, now the lab protocol must include them and determine if a preservative and which preservative is necessary or optional. Also, the type of testing determines preservative use. Radioactive immunoassay (RIA) is now outdated, but is still being used. HPLC Tandem Mass Spectrometry is the most current, with Liquid Chromatography Tandem Mass Spectrometry (LCMSMS) running a close second. Bacteria degrade the cortisol quickly at room temperature, and getting a lab to refrigerate it is tenuous. You hope they will but often they don't. Measure/aliquoting a correct sample is another problem if there is more than one jug for a collection. The aliquot must be a mix of all samples so there is a proper average and the
knol.google.com/k/cushingsdiseasesyndrome# 13/22
2/26/12
Cushing's Disease/Syndrome a knol by Robin S
TOTAL volume for all jugs used in the 24-hour collection has to be recorded. From experience and observation, I offer my opinion: Find a lab which will use one of the preferred methods above (not RIA), will refrigerate the urine until tested, will not add a preservative, and which understands all must be THAWED and MIXED before aliquoting it. If the urine aliquot/sample is sent elsewhere, it needs to be sent FROZEN to be thawed before measured, and the total amount of urine that was presented needs to be recorded. Another test that is used extensively for the measurement of cortisol, especially with diurnal variation or lack of, is serum cortisol. This one is pretty straight forward as long as the person reading the lab results understands what they really mean. The article, "Cushing's Syndrome" by John Newell-Price, Xavier Bertagna, Ashley B Grossman, Lynnette K Nieman, Lancet 2006; 367: 160517,Division of Clinical Sciences, University of Sheffi eld, Northern General Hospital, Sheffi eld, UK (J Newell-Price FRCP); says An awake midnight concentration of cortisol in plasma of more than 207 nmol/L differentiates between Cushings syndrome and other causes of hypercortisolaemia but can miss mild disease diagnosis in about 7% of cases. (The conversion factor of g/dL x 27.6 = nmol/L) Essentially, serum cortisols only have valid ranges for the 8 a.m. and 4 p.m. time periods in most labs. The research and leading endocrinologists acknowledge the validity of midnight serum cortisols, also. So, as I said before, the 8 a.m. should be the highest of the three, with the 4 p.m. about half of the 8 a.m. value. The midnight value should be close to zero. The problem is that the labs don't know about the midnight range of zero, so IF one can find a lab willing to draw a midnight serum cortisol fairly frequently for testing, they won't know to put "zero" on the range and flag it as high if it's above that. 7.5 g/dL (207 nmol/L) is diagnostically high. If you have someone ordering tests for you who does not understand this, you may get a call saying "it was normal". Ask for the actual report. A third test that is important in the diagnosing of Cushing's and differentiating between types is Adrenocorticotropic Hormone (ACTH). The lab technician must use an EDTA (lavender top) plasma tube only! Collection in nonsiliconized tubes can result in falsely low results as ACTH adheres to glass. It needs to be mixed by inversion and centrifuged immediately after collection. IT MUST NOT GET WARM. The tubes should be on ice prior to drawing the blood, and put immediately back in the ice until it is separated and then frozen. The plasma must be separated and frozen immediately. If it is shipped, it should be shipped on dry ice while still frozen. ACTH breaks down easily in heat, and if not collected and preserved on ice, proteolysis (degradation of proteins by cellular enzymes) can reduce the plasma concentration. The salivary cortisol test is another test used very similarly to the serum cortisol tests. It, too, can help show diurnal rhythm (or lack thereof). The protocol is pretty simple and straight-forward regardless of the lab. There are only a few labs who use an FDA approved test for these salivary cortisol levels. The labs are Esoterix, ACL Laboratories, LabCorp (who now owns Esoterix), and Quest. It is very easy for a doctor to set up an account with any of these and order the testing kits for patients. The patient simply collects "spit" until s/he gathers enough for the container or "salivette". Some of the labs use a tampon-like material (salivette) for the patient to soak with saliva and place in the tube. Both methods of collection are reliable. However, it is important that one does not touch the salivette with hands and should not eat, drink or rinse the mouth for thirty minutes prior to collection.
knol.google.com/k/cushingsdiseasesyndrome# 14/22
2/26/12
Cushing's Disease/Syndrome a knol by Robin S
A word of caution. Although the cortisol supposedly does not break down very quickly, many get much higher results when they first froze the samples, then shipped them overnight. Since bacteria will break down cortisol, it stands to reason this can happen with the salivary test, too. Another inconsistency with salivary cortisols that many folks testing have observed is they have had high serum cortisols the same nights we have done salivary cortisols. I did several while sitting and having blood drawn. My serum cortisol was high. My salivary cortisol was "normal". Only when my serum cortisol (at midnight) was around 16 g/dL or higher did my salivary cortisol show high levels, too. The clearance is different, thus the outcomes can vary. What advice do I have for testing? You must be proactive in your testing process. Know the proper protocols Take print-outs of the proper protocols with you. You can find them for just about any lab. If you can't, the Mayo site and ARUP labs have great details. Labcorp and Esoterix also have may good, informative articles. If your doctor wants it done a certain way, get him/her to put that in writing Insist on the proper protocol. This is YOUR TIME and YOUR MONEY (even with insurance) that is paying for a service to be done right. You may need to catch that high and you don't want anyone to mess it up. Call the lab ahead of time and speak with the lab manager. Explain your situation courteously and ask if they are familiar with the tests and protocols. Go over them together, talk about when you will be in, and what you need to do to make sure those protocols are followed. This will save you many headaches down the road. A good relationship with your lab will go a LONG way. Call the lab manager again if you do encounter problems and discuss the issues. Courtesy still goes further than anger. However, I've been known to use some of that southern "charm" my mother taught me. Ahem... For midnight serums, it is difficult to find a lab, other than the local hospital lab, open late at night. Again, call them ahead of time, explain the testing protocol and why you need to come in at midnight. Get the paperwork done ahead of time so you can go in and get it done quickly once you are there. A little kindness goes a long way. A treat, some cookies, a gift basket or something simple as a cake shared with the lab folks occasionally will brighten their day. Say thank-you when it is done right. Again, that goes a long way.
Don't be surprised if drawing blood is difficult.This is typically due to the effect of the cortisol on the veins so try to keep your arms warm and stay hydrated. Warm bags of rice, a warm fleece jacket, or whatever it takes to keep those arms warm are helpful.
Testing101:IPSS(akaBIPSS)
Once the biochemical evidence for Cushing's Disease/Syndrome confirms the diagnosis, differentiation of the source or reason for the high cortisol must be ascertained. There are numerous possible scenarios for questionable source(s). I'll try to outline a few of the more common ones. Scenario 1: Normal or high ACTH, high cortisol with abnormal circadian rhythm, no evident pituitary
knol.google.com/k/cushingsdiseasesyndrome# 15/22
2/26/12
Cushing's Disease/Syndrome a knol by Robin S
tumor on MRI or questionable hypointense area which may or may not mean a tumor, no evident adrenal tumor. **Possible sources of high cortisol: ACTH-secreting pituitary adenoma, ectopic ACTH-secreting tumor Scenario 2: Normal or high ACTH, high cortisol with abnormal circadian rhythm, visible pituitary tumor on MRI, no evident adrenal tumor. **Possible sources of high cortisol: ACTH-secreting pituitary adenoma, ectopic ACTH-secreting tumor Scenario 3: Normal ACTH, high cortisol with abnormal circadian rhythm, visible pituitary tumor on MRI, visible adrenal tumor. **Possible sources of high cortisol: ACTH-secreting pituitary adenoma, adrenal tumor There are more possible scenarios. The point I'm trying to make is that the evidence is not always cut-anddry, and the source of high cortisol needs to be determined. One tool for doing this is the Bilateral Inferior Petrosal Sinus Sampling (BIPSS) aka IPSS. In CLINICAL REVIEW: Cushings Syndrome: Important Issues in Diagnosis and Management [The Journal of Clinical Endocrinology & Metabolism 91(10):37463753, 2006 by The Endocrine Society], the authors state: ....mild to moderate hypercortisolism, a normal or slightly elevated plasma ACTH, and normokalemia has at least a 95% likelihood of having Cushings disease. In contrast, a patient with prodigious hypercortisolism, hypokalemia, and marked elevations of plasma ACTH may be more likely to have an occult ectopic ACTH-secreting tumor. Approximately 40-50% of pituitary tumors do not show on MRI, although the dynamic protocol used in some specialty centers increases the odds. Couple that with the 10% chance of an incidentaloma and diagnosticians find the need for more validation. The aforementioned authors also say "Inferior petrosal sinus ACTH sampling with CRH stimulation is the only study having the potential to yield a diagnostic sensitivity and specificity for Cushings disease higher than its pretest probability". They are talking about the IPSS. What is an IPSS? An IPSS is a test to sample the amount of ACTH draining into the inferior (vs. the superior) petrosal sinuses from the pituitary. Two catheters (one on each side) are threaded from the groin area up each side of the body to a major vein in the petrosal sinus area. Corticotropin-releasing hormone (CRH), a natural hormone secreted by the hypothalamus, naturally stimulates the anterior pituitary gland to produce ACTH. If only the pituitary, not a tumor, is secreting ACTH, the levels of ACTH in the petrosal sinus area and in the rest of the body should be very close. If the levels of ACTH in the petrosal sinus area is higher than in samples from a peripheral area of the body , then an adenoma/tumor on or close to the pituitary is probably secreting ACTH. Since CRH is released in pulses naturally and will not be active all the time, CRH is added during the IPSS to stimulate the pituitary to secrete ACTH during the testing period. After drawing blood from the catheters and from a peripheral area (arm or leg) to give a baseline level of ACTH and cortisol, CRH is injected. So that levels of ACTH are recorded from both the petrosal sinus area and the peripheral area, blood is drawn from each area at a specified interval (5-15 minutes, usually) to determine these levels over time. This
knol.google.com/k/cushingsdiseasesyndrome# 16/22
2/26/12
Cushing's Disease/Syndrome a knol by Robin S
usually takes about 2 hours. The blood samples are kept on ice and sent immediately to the lab for determination of ACTH levels. There will be three major comparisons: 1) Right petrosal sinus samples 2) Left petrosal sinus samples 3) Peripheral samples If the ratio of the right-to-peripheral sample OR the ratio of the left-to-peripheral sample is greater than 2 before the addition of CRH, the value is diagnostic for Cushing's Disease (pituitary source). If the ratio of the right-to-peripheral sample OR the ratio of the left-to-peripheral sample is greater than 3 after the addition of CRH, the value is diagnostic for Cushing's Disease (pituitary source). The IPSS may also be used tentatively for lateralization. This means it can help predict which side the tumor may be on. However, this is only about 70% accurate. For this to work, the tumor will drain more on one side than the other. However, some tumors drain into both sides, or there are multiple tumors. Sometimes, the anatomy is not predictable and the tumor may drain into the sinus on the opposite side. How valid is the IPSS? Multiple studies cite the usefulness of the IPSS in determining source, but not as a diagnostic tool. Biochemical evidence needs to show there is hypercortisolism prior to the use of the IPSS. The rate of falsepositives is relatively small, but there are false-negatives. In other words, some patients who have had proven Cushing's Disease had a negative IPSS. This is usually due to the infamous episodic/cyclic/subclinical/mild "version" of Cushing's. In the article, Petrosal sinus sampling for diagnosis of Cushing's disease: evidence of false negative results, [Clin Endocrinol (Oxf). 1996 Aug;45(2):147-56], the authors say: Only when a significant IPS:P ACTH ratio is present can Cushing's disease tbe established by IPS sampling. The absence of a significant IPS: P ACTH ratio does not necessarily imply ectopic secretion of ACTH, nor does it exclude Cushing's disease. The results of lateralization by IPS sampling do not remove the need for a thorough transsphenoidal examination of the contents of the sella turcica. In the Endocrinologist [11(5):388-398, September/October 2001] researchers at the University of Virginia say " In experienced centers, the diagnostic sensitivity and specificity of IPSS approaches 100%. The indications for IPSS are debated, with some advocating use when standard dynamic tests are inconclusive, and others advocating use only when pituitary magnetic resonance imaging (MRI) is inconclusive. "
Testing101:GrowthHormoneDeficiency
knol.google.com/k/cushingsdiseasesyndrome#
17/22
2/26/12
Cushing's Disease/Syndrome a knol by Robin S
Ok...what's the big deal, anyhow? Why would an adult need to have her growth hormone levels evaluated? As a review, growth hormone is secreted by the pituitary gland. Some pituitary tumors secrete too much GH which causes gigantism in children and acromegaly in adults. However, on the flip side, some tumors suppress the pituitary and too little is secreted. Even if the tumor does not do that, surgery to remove a tumor may cause the pituitary to quit or lessen it's secretion of GH. Radiation is also used on pituitary tumors that cannot be totally removed or if there is hyperplasia, and it, too, can damage the pituitary. Adult Growth Hormone Deficiency (GHD) is a very real problem. GH maintains a healthy balance of muscle, bones, and fat and if an adult is deficient, her body composition changes. The body has less muscle, visceral fat is deposited around the abdomen, and bones weaken. Other fats in the body are affected. "Good" cholesterol (HDL) decreases but "bad" cholesterol (LDL) increases. This is very hard on the cardiovascular system (remember, the heart is a muscle) and the cerebrovascular system. In Diagnosis of adult GH deficiency [V. Gasco, et al, Pituitary (2008) 11:121128], the authors state: Adults with growth hormone deficiency (GHD) have impaired health, which improves with GH replacement. GHD in adults leads to impairment in body composition and function, as well as to deranged lipoprotein and carbohydrate metabolism and increased cardiovascular morbidity. Based on evidence that GHD in adults is a new syndrome which may benefit from GH replacement, health authorities in many countries have approved the therapeutic use of GH in hypopituitaric patients with severe GHD. Not only is the physical health of a GHD adult affected. Social isolation, excessive tiredness, anxiety, depression, and apathy are also symptoms of GHD. Growth hormone secretion is pulsatile which means random measurements of GH levels are not helpful or diagnostic. Since insulin-like growth factor-1(IGF-1) is stimulated by GH but does not fluctuate during the day like GH, it is useful in monitoring GH levels. Low levels are an excellent indication of a GHD problem. However, normal levels do not mean there is no deficiency. The Growth Hormone Research Society met in 2007 in Australia and penned a consensus statement about the problems, testing, and treatments associated with adult GHD. In their consensus statement, they write: ...the patient with objective evidence of hypothalamicpituitary disease (e.g., on imaging or after irradiation), who may present with organic isolated GHD as the first hormonal deficiency...may account for up to 25% of cases of GHD in the adult.
knol.google.com/k/cushingsdiseasesyndrome# 18/22
2/26/12
Cushing's Disease/Syndrome a knol by Robin S
Consensus guidelines for the diagnosis and treatment of adults with GH deficiency II[European Journal of Endocrinology (2007) 157 695700] In this same consensus statement, they say: Not all patients suspected of having GHD,however, require a GH stimulation test for diagnosis.Patients with three or more pituitary hormone deficiencies and an IGF-I level below the reference range have >97% chance of being GHD, and therefore do not need a GH stimulation test. The Insulin Tolerance Test has, in the past, been the "gold-standard" for measuring true GHD. However, there have been some problems with its reproducibility and specificity. In Clinical Presentation and Diagnosis: Growth Hormone Deficiency in Adults the American Journal of Managed Care [Volume 10:S424-S430 , October 2004 , Number 13 Suppl ] states: Numerous pharmacologic agents can be used to assess GH production and secretion by the pituitary in adults (Table 3). These include insulin, arginine, levodopa (L-dopa), arginine plus L-dopa, arginine plus GHRH, and the glucagon test. None display perfect sensitivity and specificity; however, the insulin tolerance test (ITT) and arginine-GHRH are excellent tests. The arginine-GHRH test is being used by major pituitary centers around the world. It is less stressful with less risk for the patient but yields reproducible and accurate results. What are the differences in these two tests? In the ITT, the pituitary is provoked to produce GH by causing hypoglycemia in the patient with insulin. With the A-GHRH test, arginine is a somatostatin antagonist which essentially does the same thing. Combined with GHRH (to stimulate GH production), it is now the test of choice (see article linked above). Just how many kinds of tumors are there associated with Cushing's? Wait, are we talking about pituitary tumors? Or adrenal tumors? How about ectopic tumors? Are you beginning to get the picture of why this illness is so hard to diagnose?
WhattypesoftumorsareassociatedwithCushing's?
PITUITARYTUMORS/ADENOMAS:
So, let's talk about tumors. These are also sometimes called adenomas. Pituitary adenomas are classified several ways. They may be classified by pathology, by size, and by hormone production. I'm going to keep it simple here and list the basic types of tumors by the hormones they produce. Bear in mind that many pituitary adenomas produce more than one hormone. This production is not held in check by the body's normal feedback loops, thus they aren't controlled. Corticotroph (ACTH-Producing) Adenomas :
knol.google.com/k/cushingsdiseasesyndrome# 19/22
2/26/12
Cushing's Disease/Syndrome a knol by Robin S
The corticotroph adenoma secretes adrenocorticotropic hormone (ACTH), which results in Cushing Disease because it stimulates the adrenal glands to overproduce cortisol. These tumors are initially confined to the sella turcica, but they may enlarge and become invasive especially after bilateral adrenalectomy. This is called Nelson's Syndrome. Somatotroph (GH-Producing) Adenomas: Somatotroph adenomas produce growth hormone (GH), resulting in gigantism in younger patients and acromegaly in adults. These tumors may also extend beyond the sella. Thyrotroph (TSH-Producing) Adenomas: Thyrotroph adenomas secrete thyroid-stimulating hormone (TSH), also known as thyrotropin, which results in hyperthyroidism without TSH suppression. Many are large and invasive and typically have other types of adenoma cells included, such as ACTH and/or GH. Lactotroph (PRL-Producing) Adenomas: Lactotroph adenomas secrete prolactin (PRL) and are typically an intrasellar tumor. However, they can become large enough to enlarge the sella turcica. Nonfunctioning (Endocrine-Inactive) Adenomas: These tumors cause symptoms when they extend beyond the sella, which results in pressure on the surrounding structures such as optic nerves and carotid veins. They are not associated with clinical and biochemical evidence of hormone excess. Carcinomas: Pituitary carcinomas, although extremely rare, are usually endocrinologically functional, and ACTHproducing and PRL-producing tumors are the most frequent. Other Tumors: Other tumors of the pituitary include craniopharyngiomas, meningiomas, and germ cell tumors. Even rarer are the granular cell tumors, pituicytomas, and gangliogliomas. Most rare include gangliocytomas, lymphomas, astrocytomas, and ependymomas.
ADRENALTUMORS/ADENOMAS:
When a tumor in an adrenal gland overproduces hormones, the tumor is called a functioning tumor. A tumor in an adrenal gland that does not produce hormones is, understandably, called a nonfunctioning tumor. A tumor can start in an adrenal gland (called a primary adrenal tumor) or it can begin in another organ, such as the lungs, and then metastasize (spread) to the adrenal glands. I'm going to focus on primary adrenal gland tumors. Adenoma: An adenoma is a benign nonfunctioning tumor of the adrenal cortex. Also called an adrenocortical adenoma, this tumor usually does not cause symptoms, and, if it is small, may not require any treatment. However, as it grows it can put pressure on parts of the gland causing it to under or overproduce hormones. The cause of adrenal adenomas is unknown, but the current accepted theory is that they arise because of mutations in certain genes. Adrenal adenomas are more common in some inherited diseases, including multiple endocrine neoplasia type I, Beckwith-Wiedemann syndrome and the Carney complex. Chronic adrenal stimulation by ACTH leads to bilateral adrenocortical hyperplasia and, if longstanding, nodular transformation according to recent research. Thus, an ACTH producing tumor of the pituitary or ectopic tumor may stimulate the adrenals to form tumors or become hyperplastic (more about hyperplasia in a bit). Adrenocortical carcinoma:
knol.google.com/k/cushingsdiseasesyndrome# 20/22
2/26/12
Cushing's Disease/Syndrome a knol by Robin S
Although exceedingly rare this is the most common type of malignant adrenal gland tumor, affecting the cortex, also called an adrenal cortical carcinoma. Adrenocortical carcinoma can be a functioning or nonfunctioning tumor. If the tumor is functioning, it may produce more than one hormone. Pheochromocytoma: A pheochromocytoma is a rare tumor that develops in the core of an adrenal gland. It secretes excessive amounts of catecholamines, usually epinephrine and norepinephrine. Neuroblastoma: Neuroblastoma is a disease in which malignant cells form in nerve tissue of the adrenal gland. It is very rare. If that isn't enough, there is another form of tumor that isn't a tumor. It's called hyperplasia. These tumor cells may invade the pituitary or the adrenals in nests throughout the gland. Pituitary Hyperplasia: A Review Adrenal Hyperplasia To learn more: Cushing's Help and Support Cushing's Help and Support Foundation
Comments
Sign in to write a comment
hammad saleem Coronary Artery Disease: New ways of Treatment Great article Have a look at this video for more insights http://www.youtube.com/watch?v=pjUndFhJkjM Thanks Sep 22, 2011 3:52 AM Report abusive comment 0 Post reply to this comment
Internet Medical Publishing
knol.google.com/k/cushingsdiseasesyndrome# 21/22
2/26/12
Cushing's Disease/Syndrome a knol by Robin S
Invitation to submit your Knols to the journal "Open Journal of Medicine" Wed like to invite you to submit your knols to the journal "Open Journal of Medicine" by clicking the link "Submit a knol to this collection" at the bottom of this page http://knol.google.com/k/internet-medicalpublishing/open-journal-of-medicine/1bbsle13m97c0/391#view Best, iMedPub Last edited Dec 24, 2009 4:17 AM Report abusive comment 0 Post reply to this comment
knol.google.com/k/cushingsdiseasesyndrome#
22/22
Vous aimerez peut-être aussi
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- Molitch - Hot Topics Cushings Disease and AcromegalyDocument69 pagesMolitch - Hot Topics Cushings Disease and AcromegalyMaryOPas encore d'évaluation
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- Adrenal Hyper FunctionDocument1 pageAdrenal Hyper FunctionMaryOPas encore d'évaluation
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5794)
- Addison's Disease: Adrenal Insufficiency and Adrenal CrisisDocument15 pagesAddison's Disease: Adrenal Insufficiency and Adrenal CrisisMaryOPas encore d'évaluation
- Cyclic Cushing'sDocument8 pagesCyclic Cushing'sMaryOPas encore d'évaluation
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (400)
- CPR PresentationDocument33 pagesCPR Presentationathar.shahPas encore d'évaluation
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (588)
- Ensign 2.4 Pain ActivityDocument4 pagesEnsign 2.4 Pain Activitydanaensign100% (1)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- Group 10 - Time & Stress Management NotesDocument6 pagesGroup 10 - Time & Stress Management NotesMimi Lizada BhattiPas encore d'évaluation
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- Aurora 4Document8 pagesAurora 4Jesus PerezPas encore d'évaluation
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (895)
- Diabetes QuestionsDocument6 pagesDiabetes QuestionsLara B100% (3)
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- RhinosporidiosisDocument9 pagesRhinosporidiosisDrAnwar MKPas encore d'évaluation
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- FE ImbalanceDocument6 pagesFE ImbalanceDonna CortezPas encore d'évaluation
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- Dual Controlled Modes of Mechanical VentilationDocument22 pagesDual Controlled Modes of Mechanical VentilationTC Ugur KocaPas encore d'évaluation
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (266)
- Test Bank For Berry and Kohns Operating Room Technique 13th Edition by PhillipsDocument3 pagesTest Bank For Berry and Kohns Operating Room Technique 13th Edition by Phillipsa522345415100% (3)
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (74)
- American Academy of Pediatrics: ABBREVIATION. PICU, Pediatric Intensive Care UnitDocument3 pagesAmerican Academy of Pediatrics: ABBREVIATION. PICU, Pediatric Intensive Care UnitdoctorsamitPas encore d'évaluation
- Rift Valley FeverDocument9 pagesRift Valley Feverapi-390015550Pas encore d'évaluation
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- 41 Prep GuideDocument73 pages41 Prep Guidedaljit chahalPas encore d'évaluation
- Antiepileptic Drugs To Treat Psychiatric DisordersDocument435 pagesAntiepileptic Drugs To Treat Psychiatric DisordersHugo HernandezPas encore d'évaluation
- Isbar Clear CommunicationDocument1 pageIsbar Clear Communicationbapimirab654Pas encore d'évaluation
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (345)
- Continuous Monitoring of Erns: Set of Ern Core IndicatorsDocument23 pagesContinuous Monitoring of Erns: Set of Ern Core IndicatorsElenaPas encore d'évaluation
- UntitledDocument33 pagesUntitledapi-257817850Pas encore d'évaluation
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2259)
- Protocol Deviation Tracking Log Ver2!07!17-2015Document3 pagesProtocol Deviation Tracking Log Ver2!07!17-2015Kesava Sumanth APas encore d'évaluation
- Tracheotomies Bronchoscopy: Med-Surg Final Exam Study Guide Fall 2010Document3 pagesTracheotomies Bronchoscopy: Med-Surg Final Exam Study Guide Fall 2010Lynn SueningPas encore d'évaluation
- 12 Steps of Aseptic TechniqueDocument7 pages12 Steps of Aseptic TechniqueRobbie MejiaPas encore d'évaluation
- Renzo Colombo, Richard Naspro PDFDocument9 pagesRenzo Colombo, Richard Naspro PDFHidayah Mararotua HarahapPas encore d'évaluation
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1090)
- Therapeutic Drug MonitoringDocument6 pagesTherapeutic Drug MonitoringEdi Uchiha SutarmantoPas encore d'évaluation
- Calcified Cephalohematoma in A Newborn Unusual Presentation Without History of Birth TraumaDocument6 pagesCalcified Cephalohematoma in A Newborn Unusual Presentation Without History of Birth Traumafaizu21Pas encore d'évaluation
- Surgical Designs and Techniques For Mandibular Contouring Based On Categorisation of Square Face With Low Gonial Angle in OrientalsDocument8 pagesSurgical Designs and Techniques For Mandibular Contouring Based On Categorisation of Square Face With Low Gonial Angle in Orientalsfluffy4202011Pas encore d'évaluation
- Effects of Isotretinoin On Meibomian GlandsDocument6 pagesEffects of Isotretinoin On Meibomian GlandsTeddyPas encore d'évaluation
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (121)
- About MeningiomaDocument5 pagesAbout MeningiomaRPh Krishna Chandra JagritPas encore d'évaluation
- ECG MachinesDocument5 pagesECG MachinesThiagarajan KanagarajanPas encore d'évaluation
- WRP Australia 2017 Case Study-1newDocument10 pagesWRP Australia 2017 Case Study-1newRohmatullahPas encore d'évaluation
- Diadynamic CurrentDocument30 pagesDiadynamic Currentpartizan_bl100% (2)
- Two-Unit Cantilevered Resin-Bonded Fixed Partial Denture As A Substitute For A Prosthodontic-Orthodontic Treatment Plan: A 5-Year Case ReportDocument7 pagesTwo-Unit Cantilevered Resin-Bonded Fixed Partial Denture As A Substitute For A Prosthodontic-Orthodontic Treatment Plan: A 5-Year Case ReportMaika RatriPas encore d'évaluation
- CQCInvestigation July 2010Document40 pagesCQCInvestigation July 2010Nestor WebmasterPas encore d'évaluation
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)