Académique Documents
Professionnel Documents
Culture Documents
Patent Ductus Arteriosus
Transféré par
nidyaparasayuDescription originale:
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Patent Ductus Arteriosus
Transféré par
nidyaparasayuDroits d'auteur :
Formats disponibles
PATENT DUCTUS ARTERIOSUS PDA
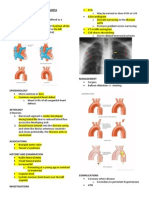
Patent ductus arteriosus (PDA) is a condition in which the ductus arteriosus does not close. (The word "patent" means open.) The ductus arteriosus is a blood vessel that allows blood to go around the baby's lungs before birth. Soon after the infant is born and the lungs fill with air, the ductus arteriosus is no longer needed. It usually closes in a couple of days after birth. PDA leads to abnormal blood flow between the aorta and pulmonary artery, two major blood vessels that carry blood from the heart.
Anatomy The ligamentum arteriosum is a piece of fibrous tissue that develops from the ductusarteriosus (DA). It takes shape within three weeks of a person's birth and remains a feature in the normal adult heart. The term ligamentum arteriosum is the Latin phrase for the arterialligament. Some people describe the ligamentum arteriosum as a non-functional bit of the ductusarteriosus after embryonic formation. Others, however, cite the vestige as contributing to the rupture of the aorta the body's largest artery during major trauma. This is because it keeps the artery in place when it springs back to its original position during a rapid deceleration. As a result, the aorta may break open. During prenatal development, the heart of the fetus has a small hole or passage called the ductus arteriosus. Also called the ductus Botalli, it connects the pulmonary artery, which transports deogygenated blood from the heart to the lungs and the area of the aorta, referred to as the aortic arch. In the fetus, however, the DA is meant for permitting a significant amount of blood from the heart's right ventricle to pass around the fluid-filled lungs. Within 12 to 24 hours after birth, however, the DA begins to close a process that is typically completed within three weeks. The ligamentum arteriosum is what is left after this closure, and is connected to the the aortic arch and the pulmonary artery. The pulmonary artery is located to the arterial ligament's inferomedial, or lower middle, area. The aortic arch is posterosuperior to the ligament, or located at its back and above it. Lateral to the ligamentum arteriosum is the vagus nerve, which is the 10th of the 12 pairedcranial nerves. For this reason, it is also called cranial nerve X. Also lateral to the vestige is the recurrent laryngeal nerve, or Galen's nerve, that springs from the vagus nerve on the left. The laryngeal nerve hooks around the ligament from the rear where part of the aorta is located, then it goes up to the voice box after which it is named. Anteriorally, or frontally, the ligamentum arteriosum is near the surface of a network of nerves that innervate the heart from its base. The tangle is called the cardiac plexus. Also to the front of the ligamentum arteriosum is the phrenic nerve, which mainly emerges from the fourth cervical nerve (C4).
Causes, incidence, and risk factors PDA affects girls more often than boys. The condition is more common in premature infants and those with neonatal respiratory distress syndrome. Infants with genetic disorders, such as Down syndrome, and whose mothers had rubella during pregnancy are at higher risk for PDA. PDA is common in babies with congenital heart problems, such as hypoplastic left heart syndrome, transposition of the great vessels, and pulmonary stenosis. Pathology The ductus arteriosus originates embryologicaly from the left aortic arch artery therefore it connects the left pulmonary artery to the aortic arch opposite to the left subclavian artery. Patent ductus arteriosus when it closes it usually does so beginning from the pulmonary artery end, leaving a diverticulum in the aortic site which eventually closes. The patent ductus arteriosus goes into the left pulmonary artery even in right aortic arch. Rarely does it originates from the right pulmonary artery. In tetralogy of Fallot the patent ductus arteriosus is usually absent or small. In pulmonary atresia the patent ductus arteriosus is small because in-utero the blood shunts left-to-right in small volumes to the lungs. In premature babies the patent ductus arteriosus closes later than it would in term babies. However, if the prematurity is taken into consideration it closes about the same time. Patent ductus arteriosus closes with high oxygen tension which explains why at high altitude the patent ductus arteriosus remain patent for a longer time. Oxygen concentration above atmospheric level does not assist in closing the patent ductus arteriosus faster then it would naturally. The etiology of higher incidence of patent ductus arteriosus in maternal rubella infection is not clear but it could be due to tissue changes in the ductus arteriosus similar to those observed in right pulmonary artery and left pulmonary artery branches. Indomethicin if given to the mother at pre-term will cause the patent ductus arteriosus to constrict. This is due to interference with the arachidonic derivative causing lack of Prostaglandin. Supplemental oxygen will cause the patent ductus arteriosus to close in full term but not in premature babies. On the other hand,
prostaglandin is better at keeping the patent ductus arteriosus open in premature babies versus full term babies. Polycythemia in patent ductus arteriosus with pulmonary vascular obstructive disease and right-to-left shunting tends to be more so than other cyanotic congenital heart disease. This is thought to be because of increased cyanotic blood flow to the kidneys triggering the mechanism for polycythemia.
Pathopysiology
The murmur in patent ductus arteriosus is characterized as being systolic and diastolic with a crescendo and decrescendo pattern, peaking at around the closure of the aortic valve (A2). Due to the left-to-right shunting at the patent ductus arteriosus the pulmonary venous return will be increased causing dilatation of the left atrium and stretching of the patent foramen ovale with more left-to-right shunting at the atrial level worsening congestive heart failure. The patent ductus arteriosus murmur is best heard over the second left intercostal space. Clicking noises during the murmur gives the characteristic machinery quality of the patent ductus arteriosus murmur. Murmurs mimicking patent ductus arteriosus include AP window, venous hum, ruptured sinus of Valsalva (aorta to RA, RV or LA shunts), coronary artery to ventricular cavity fistula and tetralogy of Fallot with pulmonary atresia and large collaterals. Patients present with congestive heart failure, this include easy fatigability (poor feeding in infants), shortness of breath, pallor sweating and cool extremities with exertion. Signs include bounding pulses, increase left ventricular apical impulse, thrill, continuous murmur and pulmonary sounds consistent with pulmonary edema such as rales and wheezing.
Symptoms A small PDA may not cause any symptoms. However, some infants may have symptoms such as:
Fast breathing Poor feeding habits Rapid pulse Shortness of breath Sweating while feeding
Tiring very easily Poor growth
Signs and tests Babies with PDA often have a heart murmur that can be heard with a stethoscope. However, in premature infants, a heart murmur may not be heard. The health care provider may suspect the condition if the infant has breathing or feeding problems soon after birth. Changes may be seen on chest x-rays. The diagnosis is confirmed with an echocardiogram. Sometimes, a small PDA may not be diagnosed until later in childhood. Treatment If the rest of the baby's heart and blood flow is normal or close to normal, the goal is to close the PDA. If the baby has certain other heart problems or defects, keeping the ductus arteriosus open may be lifesaving. Medicine may be used to stop it from closing. Sometimes, a PDA may close on its own. In premature babies it often closes within the first 2 years of life. In full-term infants, a PDA rarely closes on its own after the first few weeks. When treatment is needed, medications such as indomethacin or a special form of ibuprofen are often the first choice. Medicines can work very well for some newborns, with few side effects. The earlier treatment is given, the more likely it is to succeed. If these measures do not work or can't be used, the baby may need to have a medical procedure. A transcatheter device closure is a procedure that uses a thin, hollow tube placed into a blood vessel. The doctor passes a small metal coil or other blocking device through the catheter to the site of the PDA. This blocks blood flow through the vessel. These coils can help the baby avoid surgery. Surgery may be needed if the catheter procedure does not work or it cannot be used. Surgery involves making a small cut between the ribs to repair the PDA. Surgery has risks, however. Weigh the possible benefits and risks with your health care provider before choosing surgery.
Expectations (prognosis) If a small PDA stays open, the baby may eventually develop heart symptoms. Babies with a larger PDA could develop heart problems such as heart failure, high blood pressure in the arteries of the lungs, or an infection of the inner lining of the heart if the PDA does not close Reference 1. Webb GD, Smallhorn JF, Therrien J, Redington AN. Congenital heart disease. In: Bonow RO, Mann DL, Zipes DP, Libby P, eds. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine, 9th ed. Philadelphia, Pa:Saunders Elsevier; 2011:chap 65.
Vous aimerez peut-être aussi
- Congenital Heart Disease - Cynotic AcynoticDocument34 pagesCongenital Heart Disease - Cynotic Acynoticvruttika parmarPas encore d'évaluation
- Rheumatic Heart DiseaseDocument22 pagesRheumatic Heart DiseasemajdPas encore d'évaluation
- Cardiac Output and Hemodynamic MeasurementDocument29 pagesCardiac Output and Hemodynamic Measurementdeepa100% (1)
- FinalDocument56 pagesFinalvamshidhPas encore d'évaluation
- Congestive Heart FailureDocument20 pagesCongestive Heart FailurehuzaifahjusohPas encore d'évaluation
- Acquired Heart DiseasesDocument41 pagesAcquired Heart DiseasesSaman SarKoPas encore d'évaluation
- ECG in Emergency MedicineDocument228 pagesECG in Emergency MedicineBobbyGustafsonPas encore d'évaluation
- Cardiac Cycle - DR Rakesh JainDocument97 pagesCardiac Cycle - DR Rakesh JainEmmiePas encore d'évaluation
- Cardio-Vascular Disease: Mitral Stenosis & Mitral RegurgitationDocument25 pagesCardio-Vascular Disease: Mitral Stenosis & Mitral Regurgitationyulia silviPas encore d'évaluation
- BBB EcgDocument33 pagesBBB EcgLokbre YoasPas encore d'évaluation
- Arterial LinesDocument9 pagesArterial LinesRei IrincoPas encore d'évaluation
- AsphyxiaDocument35 pagesAsphyxiaAna Cristina Montillano100% (1)
- Eisenmenger SyndromeDocument6 pagesEisenmenger SyndromeWarkah SanjayaPas encore d'évaluation
- Tachyarrhythmias When Should I Refer?Document25 pagesTachyarrhythmias When Should I Refer?Cindy Seow Sieng TengPas encore d'évaluation
- ASD Atrial Septal Defect PDFDocument9 pagesASD Atrial Septal Defect PDFAco AjjahPas encore d'évaluation
- Congenital Heart DiseaseDocument6 pagesCongenital Heart DiseaseSamah KhanPas encore d'évaluation
- Episode 86 - HyperkalemiaDocument7 pagesEpisode 86 - HyperkalemiaAlok yadav100% (1)
- Valve SurgeriesDocument13 pagesValve SurgeriesShreeja SajitPas encore d'évaluation
- Cyanotic CHDDocument64 pagesCyanotic CHDKUMARAVELPas encore d'évaluation
- Breathing and Gas Exchange PowerpointDocument53 pagesBreathing and Gas Exchange Powerpointapi-263357086100% (1)
- Development of HeartDocument15 pagesDevelopment of Heartash ashPas encore d'évaluation
- AsdaDocument42 pagesAsdaratchagarajaPas encore d'évaluation
- MVR CabgDocument57 pagesMVR CabgRoshani sharmaPas encore d'évaluation
- Fetal and Neonatal ArrhthmiasDocument59 pagesFetal and Neonatal ArrhthmiasRihab Hafiz Othman100% (1)
- Procedure For Patent Ductus Arteriosus (PDA) Device ClosureDocument13 pagesProcedure For Patent Ductus Arteriosus (PDA) Device ClosurejaganjaggiPas encore d'évaluation
- Congenital Heart DiseaseDocument74 pagesCongenital Heart DiseaseKeith LajotPas encore d'évaluation
- Lung Protective Mechanical Ventilation StrategiesDocument4 pagesLung Protective Mechanical Ventilation StrategiesAnne Julia AgustinPas encore d'évaluation
- Organophosphorus Poisoning PDFDocument5 pagesOrganophosphorus Poisoning PDFEliuth Zamora100% (1)
- PTMC Percutaneous Balloon Mitral Valvuloplasty (PBMV) .NicvdDocument42 pagesPTMC Percutaneous Balloon Mitral Valvuloplasty (PBMV) .NicvdNavojit Chowdhury100% (1)
- Procedure For Balloon Aortic Valvuloplasty (BAV)Document17 pagesProcedure For Balloon Aortic Valvuloplasty (BAV)jaganjaggi100% (1)
- Cardiac ValveDocument12 pagesCardiac ValveHellen WestgrovePas encore d'évaluation
- Coarctation of The AortaDocument2 pagesCoarctation of The AortaDavid Cheng0% (1)
- Tetralogy of FallotDocument24 pagesTetralogy of FallotjustinahorroPas encore d'évaluation
- Cardiac Stress TestingDocument24 pagesCardiac Stress TestingRhoda Dela Torre ContrerasPas encore d'évaluation
- Pediatric Cardiology LectureDocument87 pagesPediatric Cardiology LectureMena HashemPas encore d'évaluation
- Heart Lung Machine PumpDocument31 pagesHeart Lung Machine Pumpmapas_salem100% (2)
- Causes of Hearing LossDocument2 pagesCauses of Hearing LossAdjei Fosu KennedyPas encore d'évaluation
- Myocardial Infarction MIDocument23 pagesMyocardial Infarction MIأبو محمدPas encore d'évaluation
- Heart Sounds: They Are The Sounds Produced by The Mechanical Activities of The Heart During Each Cadiac CycleDocument19 pagesHeart Sounds: They Are The Sounds Produced by The Mechanical Activities of The Heart During Each Cadiac Cyclevishnudurga100% (1)
- L-R ShuntDocument88 pagesL-R ShuntnanohaniwiekoPas encore d'évaluation
- Complete Atrioventricular Canal (CAVC)Document4 pagesComplete Atrioventricular Canal (CAVC)vivid1980Pas encore d'évaluation
- Neonatal and Pediatric Guidelines Arrhythmia ManagementDocument16 pagesNeonatal and Pediatric Guidelines Arrhythmia ManagementAkhmad HidayatPas encore d'évaluation
- Mitral StenosisDocument17 pagesMitral Stenosispriyanka bhowmikPas encore d'évaluation
- Lung Mechanics and Ventilation-Lecture NotesDocument16 pagesLung Mechanics and Ventilation-Lecture NotesCarlos Eduardo LinaresPas encore d'évaluation
- Bronchial AsthmaDocument21 pagesBronchial AsthmaMoonPas encore d'évaluation
- Ecg BSTDocument204 pagesEcg BSTAnusha Verghese100% (1)
- DR Sandeep - EISENMENGER SYNDROMEDocument81 pagesDR Sandeep - EISENMENGER SYNDROMEAlexandrescuPas encore d'évaluation
- Cardiac PacingDocument89 pagesCardiac Pacingsandwhale056Pas encore d'évaluation
- Cardiovascular NotesDocument23 pagesCardiovascular NotesEmily DongPas encore d'évaluation
- Post Op CardiacDocument7 pagesPost Op CardiacsimplyputmonicPas encore d'évaluation
- Cardiac Pacemakers NewDocument108 pagesCardiac Pacemakers NewAlif Fanharnita BrilianaPas encore d'évaluation
- Aortic GuidelinesDocument62 pagesAortic GuidelinesAmy Hoo Hui MayPas encore d'évaluation
- Cardiac AsthmaDocument12 pagesCardiac AsthmaNeupane KsabPas encore d'évaluation
- Anti T.B DrugsDocument120 pagesAnti T.B DrugsromalaramPas encore d'évaluation
- Cardiac Cycle: Mechanical Event and Their Electrical and Clinical CorrelationDocument28 pagesCardiac Cycle: Mechanical Event and Their Electrical and Clinical Correlationhawas muhammed100% (1)
- Valvular Heart Disease 2Document46 pagesValvular Heart Disease 2Topea BogdanPas encore d'évaluation
- Atrial Septal DefectDocument12 pagesAtrial Septal DefectNurruhaizi Aizi100% (1)
- Embryology Cardiovascula Rsystem DevelopmentDocument128 pagesEmbryology Cardiovascula Rsystem DevelopmentGhaidaa SadeqPas encore d'évaluation
- ABG InterpretationDocument10 pagesABG InterpretationNisha MathewPas encore d'évaluation
- Cabasisi - Act Sheet 1 - Nervous SystemDocument38 pagesCabasisi - Act Sheet 1 - Nervous SystemJocelyn CabasisiPas encore d'évaluation
- ER تجميعة 3Document27 pagesER تجميعة 3lclkPas encore d'évaluation
- Drug Study LCPDocument3 pagesDrug Study LCPalleen_viaPas encore d'évaluation
- Anestesi Laparoskopi Obgyn 123Document27 pagesAnestesi Laparoskopi Obgyn 123Riko RIKPas encore d'évaluation
- Overview of Hypertension in Adults - Uptodate FreeDocument32 pagesOverview of Hypertension in Adults - Uptodate FreedrsadafrafiPas encore d'évaluation
- Pathophysiology Total Anterior Circulation Infarction Left Middle Cerebral Artery (TACILMCA)Document2 pagesPathophysiology Total Anterior Circulation Infarction Left Middle Cerebral Artery (TACILMCA)PATHOSHOPPE100% (1)
- Olive Leaf ExtractDocument1 pageOlive Leaf ExtractGonzalo BasPas encore d'évaluation
- Biology Form 4 KSSM Chapter 10Document16 pagesBiology Form 4 KSSM Chapter 10Nik Husna100% (2)
- Hovaguimian DysautonomiaDocument21 pagesHovaguimian DysautonomiaAnonymous tG35SYROzEPas encore d'évaluation
- Course Name: Biomedical Instrumentation Course Code: MC1652: Wednesday, March 30, 2016 1Document214 pagesCourse Name: Biomedical Instrumentation Course Code: MC1652: Wednesday, March 30, 2016 1Sachin GuptaPas encore d'évaluation
- Aurelio, Lyca Mae Chapter 4 Lesson 2 Activity 1 & 2Document3 pagesAurelio, Lyca Mae Chapter 4 Lesson 2 Activity 1 & 23D - AURELIO, Lyca Mae M.Pas encore d'évaluation
- Ten Rules For The Management of Moderate and Severe Traumatic Brain Injury During Pregnancy: An Expert ViewpointDocument11 pagesTen Rules For The Management of Moderate and Severe Traumatic Brain Injury During Pregnancy: An Expert ViewpointKaren Campos GonzalezPas encore d'évaluation
- Summary of ECG AbnormalitiesDocument8 pagesSummary of ECG AbnormalitiesChristine Nancy NgPas encore d'évaluation
- Top 10 Papers in Dyslipidemias 2023Document3 pagesTop 10 Papers in Dyslipidemias 2023Bubu ToPas encore d'évaluation
- 12SL ECG Analysis Program eDocument4 pages12SL ECG Analysis Program eLuckman GrassrootPas encore d'évaluation
- A Modified Anatomical-Functional-Rope (Af-Rope) Score Improves Patient Selection For Patent Foramen Ovale ClosureDocument4 pagesA Modified Anatomical-Functional-Rope (Af-Rope) Score Improves Patient Selection For Patent Foramen Ovale ClosureDessytha NathaniaPas encore d'évaluation
- Hyperkalemia: Pathophysiology, Risk Factors and ConsequencesDocument10 pagesHyperkalemia: Pathophysiology, Risk Factors and Consequences李德富Pas encore d'évaluation
- 07.03.09 Chest PhysiotherapyDocument10 pages07.03.09 Chest PhysiotherapyMuhammad Fuad MahfudPas encore d'évaluation
- IM HandbookDocument430 pagesIM HandbookPhil ChanPas encore d'évaluation
- Obstetrics - MCQ - 3rd - BHMS - (Old, New, 2015)Document25 pagesObstetrics - MCQ - 3rd - BHMS - (Old, New, 2015)Anil kadamPas encore d'évaluation
- Clincal Case Presentation 5Document32 pagesClincal Case Presentation 5api-635948254Pas encore d'évaluation
- UrosepsisDocument35 pagesUrosepsisdrasas690Pas encore d'évaluation
- Branch Retinal Vein Occlusion (BRVO)Document4 pagesBranch Retinal Vein Occlusion (BRVO)Mahmoud Ahmed MahmoudPas encore d'évaluation
- Ileus Obstruksi - Anggi Eka ForendaDocument12 pagesIleus Obstruksi - Anggi Eka ForendaNadya ZahraPas encore d'évaluation
- ECG SimulatorDocument4 pagesECG Simulatorsales2581Pas encore d'évaluation
- Laryngitis and PericarditisDocument17 pagesLaryngitis and Pericarditis2B-4- TUNAC, Avvy Charlotte R.Pas encore d'évaluation
- Practical Clinical Pharmacy II - Lab. 4Document6 pagesPractical Clinical Pharmacy II - Lab. 4Ali AbrahimPas encore d'évaluation
- Coronary Artery DiseaseDocument25 pagesCoronary Artery DiseaseFaiz ShakhihPas encore d'évaluation
- Sickle Cell DiseaseDocument71 pagesSickle Cell Diseasenighat khanPas encore d'évaluation
- Skin Rash Station Worksheet and HandoutDocument5 pagesSkin Rash Station Worksheet and HandoutLou EscobarPas encore d'évaluation
- The Obesity Code: Unlocking the Secrets of Weight LossD'EverandThe Obesity Code: Unlocking the Secrets of Weight LossÉvaluation : 4 sur 5 étoiles4/5 (6)
- The Age of Magical Overthinking: Notes on Modern IrrationalityD'EverandThe Age of Magical Overthinking: Notes on Modern IrrationalityÉvaluation : 4 sur 5 étoiles4/5 (28)
- By the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsD'EverandBy the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsPas encore d'évaluation
- Think This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeD'EverandThink This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeÉvaluation : 2 sur 5 étoiles2/5 (1)
- ADHD is Awesome: A Guide to (Mostly) Thriving with ADHDD'EverandADHD is Awesome: A Guide to (Mostly) Thriving with ADHDÉvaluation : 5 sur 5 étoiles5/5 (2)
- LIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionD'EverandLIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionÉvaluation : 4 sur 5 étoiles4/5 (404)
- Raising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsD'EverandRaising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsÉvaluation : 5 sur 5 étoiles5/5 (1)
- Summary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisD'EverandSummary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisÉvaluation : 4.5 sur 5 étoiles4.5/5 (42)
- Summary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedD'EverandSummary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedÉvaluation : 5 sur 5 étoiles5/5 (81)
- The Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsD'EverandThe Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsÉvaluation : 3.5 sur 5 étoiles3.5/5 (3)
- Outlive: The Science and Art of Longevity by Peter Attia: Key Takeaways, Summary & AnalysisD'EverandOutlive: The Science and Art of Longevity by Peter Attia: Key Takeaways, Summary & AnalysisÉvaluation : 4 sur 5 étoiles4/5 (1)
- Why We Die: The New Science of Aging and the Quest for ImmortalityD'EverandWhy We Die: The New Science of Aging and the Quest for ImmortalityÉvaluation : 4 sur 5 étoiles4/5 (3)
- The Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaD'EverandThe Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaÉvaluation : 4.5 sur 5 étoiles4.5/5 (266)
- The Comfort of Crows: A Backyard YearD'EverandThe Comfort of Crows: A Backyard YearÉvaluation : 4.5 sur 5 étoiles4.5/5 (23)
- Sleep Stories for Adults: Overcome Insomnia and Find a Peaceful AwakeningD'EverandSleep Stories for Adults: Overcome Insomnia and Find a Peaceful AwakeningÉvaluation : 4 sur 5 étoiles4/5 (3)
- Love Life: How to Raise Your Standards, Find Your Person, and Live Happily (No Matter What)D'EverandLove Life: How to Raise Your Standards, Find Your Person, and Live Happily (No Matter What)Pas encore d'évaluation
- When the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisD'EverandWhen the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisÉvaluation : 3.5 sur 5 étoiles3.5/5 (2)
- Gut: the new and revised Sunday Times bestsellerD'EverandGut: the new and revised Sunday Times bestsellerÉvaluation : 4 sur 5 étoiles4/5 (392)
- Cult, A Love Story: Ten Years Inside a Canadian Cult and the Subsequent Long Road of RecoveryD'EverandCult, A Love Story: Ten Years Inside a Canadian Cult and the Subsequent Long Road of RecoveryÉvaluation : 4 sur 5 étoiles4/5 (44)
- Dark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.D'EverandDark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.Évaluation : 4.5 sur 5 étoiles4.5/5 (110)
- The Marshmallow Test: Mastering Self-ControlD'EverandThe Marshmallow Test: Mastering Self-ControlÉvaluation : 4.5 sur 5 étoiles4.5/5 (58)
- Mindset by Carol S. Dweck - Book Summary: The New Psychology of SuccessD'EverandMindset by Carol S. Dweck - Book Summary: The New Psychology of SuccessÉvaluation : 4.5 sur 5 étoiles4.5/5 (328)
- Dark Psychology: Learn To Influence Anyone Using Mind Control, Manipulation And Deception With Secret Techniques Of Dark Persuasion, Undetected Mind Control, Mind Games, Hypnotism And BrainwashingD'EverandDark Psychology: Learn To Influence Anyone Using Mind Control, Manipulation And Deception With Secret Techniques Of Dark Persuasion, Undetected Mind Control, Mind Games, Hypnotism And BrainwashingÉvaluation : 4 sur 5 étoiles4/5 (1138)
- Troubled: A Memoir of Foster Care, Family, and Social ClassD'EverandTroubled: A Memoir of Foster Care, Family, and Social ClassÉvaluation : 4.5 sur 5 étoiles4.5/5 (26)