Académique Documents
Professionnel Documents
Culture Documents
Pathophysiology of Type 2 Diabetes
Transféré par
EdwardPaulMadronio-NovedaDescription originale:
Titre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Pathophysiology of Type 2 Diabetes
Transféré par
EdwardPaulMadronio-NovedaDroits d'auteur :
Formats disponibles
Pathophysiology and Pathogenesis of Type 2 Diabetes Type 2 diabetes is the most common form of diabetes, with more than
90% of diabetics being Type 2, and 5%-10% being Type 1. Type 2 diabetes mellitus is a heterogeneous disorder with varying prevalence among different ethnic groups. In the United States the populations most affected are Native Americans, particularly in the desert Southwest, Hispanic-Americans, African-Americans, and AsianAmericans. However, Caucasian-Americans are also affected, but not at the same disproportionate percentage levels. The pathophysiology of Type 2 diabetes mellitus is characterized by peripheral insulin resistance (insulin insensitivity), cell damage, impaired regulation of hepatic glucose production, and later on: declining beta () cell function, eventually leading to possible -cell failure. The primary events are believed to be an initial insensitivity of insulin resulting in peripheral insulin resistance; and, later on, relative insulin deficiency. The key message here is that the key cellular dysfunction that occurs in Type 2 diabetes is not due to the cells (as in Type 1 diabetes) -- it's the muscle, liver, and fat cells, and also the damage to the red blood cells (due to glycation)! Author's Note: During my medical workshops with doctors, nurses, and other healthcare professionals, they were shocked to discover that cell dysfunction is NOT the primary issue with Type 2 diabetics -- especially when I show them the data from thousands of Type 2 diabetics. In fact, I was one of those Type 2 diabetics who was put on a drug protocol of 4 insulin shots a day because the doctors believed I was either a Type 1 diabetic, or a Type 2 diabetic with cell dysfunction. But, because of my biochemistry background I knew enough to ask for specific blood/urine/hormone tests that verified that I wasn't Type 1 and didn't have beta cell dysfunction, i.e. insulin serum test, c-peptide, urine ketone test, hemoglobin A1C, glutamic acid decarboxylase (GAD) antibody tests, islet cell antibody (ICA) tests, insulin antibody tests, GTT. Unfortunately, most Type 2 diabetics don't know this, and are led to take diabetic drugs that don't really help in the long run. If I had not recognized this discrepancy I would still be diabetic today and I would be taking even more insulin.
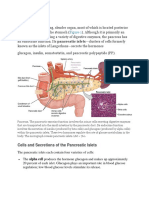
Insulin is the principal hormone that regulates uptake of glucose from the blood into most cells (primarily muscle and fat cells, but not central nervous system cells). Therefore, deficiency of insulin or the insensitivity of its receptors plays a central role in all forms of diabetes mellitus. Humans are capable of digesting some carbohydrates, in particular those most common in food; starch, and some disaccharides such as sucrose, are converted within a few hours to simpler
forms, most notably the monosaccharide glucose, the principal carbohydrate energy source used by the body. The rest are passed on for processing by gut flora largely in the colon. Insulin is released into the blood by beta cells (-cells), found in the islets of Langerhans in the pancreas, in response to rising levels of blood glucose, typically after eating. Insulin is used by about twothirds of the body's cells to absorb glucose from the blood for use as fuel, for conversion to other needed molecules, or for storage. Insulin is also the principal control signal for conversion of glucose to glycogen for internal storage in liver and muscle cells. Lowered glucose levels result both in the reduced release of insulin from the -cells and in the reverse conversion of glycogen to glucose when glucose levels fall. This is mainly controlled by the hormone glucagon, which acts in the opposite manner to insulin. Glucose thus forcibly produced from internal liver cell stores (as glycogen) re-enters the bloodstream; muscle cells lack the necessary export mechanism. Normally, liver cells do this when the level of insulin is low (which normally correlates with low levels of blood glucose). Higher insulin levels increase some anabolic ("building up") processes, such as cell growth and duplication, protein synthesis, and fat storage. Insulin (or its lack) is the principal signal in converting many of the bidirectional processes of metabolism from a catabolic to an anabolic direction, and vice versa. In particular, a low insulin level is the trigger for entering or leaving ketosis (the fat-burning metabolic phase). If the amount of insulin available is insufficient, if cells respond poorly to the effects of insulin (insulin insensitivity or resistance), or if the insulin itself is defective, then glucose will not have its usual effect, so it will not be absorbed properly by those body cells that require it, nor will it be stored appropriately in the liver and muscles. The net effect is persistent high levels of blood glucose, poor protein synthesis, and other metabolic derangements, such as acidosis. When the glucose concentration in the blood is raised beyond its renal threshold (about 10 mmol/L, although this may be altered in certain conditions, such as pregnancy), reabsorption of glucose in the proximal renal tubuli is incomplete, and part of the glucose remains in the urine (glycosuria). This increases the osmotic pressure of the urine and inhibits reabsorption of water by the kidney, resulting in increased urine production (polyuria) and increased fluid loss. Lost blood volume will be replaced osmotically from water held in body cells and other body compartments, causing dehydration and increased thirst.
Vous aimerez peut-être aussi
- Pancreatic Hormones and The Treatment of Diabetes MellitusDocument46 pagesPancreatic Hormones and The Treatment of Diabetes MellitusasmaPas encore d'évaluation
- DM Grand Case PresDocument24 pagesDM Grand Case PresBing Howell de GuzmanPas encore d'évaluation
- Diabetes Facts: Types, Causes and Symptoms ExplainedDocument13 pagesDiabetes Facts: Types, Causes and Symptoms ExplainedShelly_Ann_Del_9959Pas encore d'évaluation
- Destruction of Alpha and Beta Cells of The PancreasDocument6 pagesDestruction of Alpha and Beta Cells of The PancreasMelodia Turqueza GandezaPas encore d'évaluation
- Diabetic Coma in Type 2 Diabetes ExplanationDocument8 pagesDiabetic Coma in Type 2 Diabetes ExplanationCrystal Ann Monsale TadiamonPas encore d'évaluation
- Diabetes MellitusDocument150 pagesDiabetes MellitusIan Mizzel A. DulfinaPas encore d'évaluation
- What is diabetes? Types, symptoms and complicationsDocument11 pagesWhat is diabetes? Types, symptoms and complicationsTonee Marie Gabriel100% (1)
- Pancreas: Cells and Secretions of The Pancreatic IsletsDocument8 pagesPancreas: Cells and Secretions of The Pancreatic IsletsansahPas encore d'évaluation
- Case Study - Diabetes Mellitus (Nutrition&Dietetics)Document10 pagesCase Study - Diabetes Mellitus (Nutrition&Dietetics)Summer Suarez100% (1)
- Insulin Resistance.:) DocxDocument2 pagesInsulin Resistance.:) DocxHerbert Shirov Tendido SecurataPas encore d'évaluation
- Solutions to Diabetes and Hypoglycemia (Translated): How to prevent and get rid of it in a natural way, without resorting to medicines but adopting a correct way of lifeD'EverandSolutions to Diabetes and Hypoglycemia (Translated): How to prevent and get rid of it in a natural way, without resorting to medicines but adopting a correct way of lifePas encore d'évaluation
- Diabetes and PregnancyDocument49 pagesDiabetes and PregnancyCHRISTIAN SIDAYAPas encore d'évaluation
- Appendix B Pathophysiology of DM Type 2Document20 pagesAppendix B Pathophysiology of DM Type 2Jennifer AlvarezPas encore d'évaluation
- Pleg CheckDocument46 pagesPleg CheckSehrish BajwaPas encore d'évaluation
- Pathophysilogyof DMDocument13 pagesPathophysilogyof DMariaPas encore d'évaluation
- Aims and ObjectivesDocument13 pagesAims and Objectivesmamun183Pas encore d'évaluation
- How EXERCISE Helps Control DiabetesDocument23 pagesHow EXERCISE Helps Control DiabetesApurba MukherjeePas encore d'évaluation
- Diabetes Mellitus-1Document17 pagesDiabetes Mellitus-1JjhjPas encore d'évaluation
- Pathophysiology and Pathogenesis of Type 2 DiabetesDocument8 pagesPathophysiology and Pathogenesis of Type 2 DiabetesGladys SorianoPas encore d'évaluation
- DM Report1Document16 pagesDM Report1Wendy EscalantePas encore d'évaluation
- What Is Diabete1Document134 pagesWhat Is Diabete1Icha AjaPas encore d'évaluation
- GI and Reproductive HormoneDocument74 pagesGI and Reproductive Hormonepranutan739Pas encore d'évaluation
- Glucose Homeostasis and DiabetesDocument33 pagesGlucose Homeostasis and DiabetesSarahPas encore d'évaluation
- Diabetes AssignmentDocument8 pagesDiabetes AssignmentMildred Obeng AgyeiwaaPas encore d'évaluation
- Pancreatic Hormones Regulate Blood GlucoseDocument10 pagesPancreatic Hormones Regulate Blood Glucosekrizzia raymundoPas encore d'évaluation
- Disorder of Carbohydrate Metabolism-1Document47 pagesDisorder of Carbohydrate Metabolism-1Anggun s longgiPas encore d'évaluation
- Molecular Basis of Type 2 DiabetesDocument22 pagesMolecular Basis of Type 2 DiabetespimpellerPas encore d'évaluation
- Endocrine Week 2Document18 pagesEndocrine Week 2Clauvinna Lie FiscaPas encore d'évaluation
- Diabetes Mellitus With Hypertension (A Case Analysis)Document8 pagesDiabetes Mellitus With Hypertension (A Case Analysis)Phoespha MayangsariePas encore d'évaluation
- Pancreatic HormonesDocument41 pagesPancreatic HormonesBodea Doru100% (1)
- JKLKJKJ 113416Document13 pagesJKLKJKJ 113416Nilabha ChakrabortyPas encore d'évaluation
- Lecture - 2015 03 17Document28 pagesLecture - 2015 03 17Chanchal 12Pas encore d'évaluation
- Presentation On Diabetes Mellitus in Pharmacology LabDocument35 pagesPresentation On Diabetes Mellitus in Pharmacology Labpurwo yuhantoPas encore d'évaluation
- Biochem Lec Term Paper HypoglycemiaDocument8 pagesBiochem Lec Term Paper Hypoglycemiaapi-318284296Pas encore d'évaluation
- Diabetes Millitus PDFDocument41 pagesDiabetes Millitus PDFAbdullah BhattiPas encore d'évaluation
- Risk Factors For Diabetes MellitusDocument16 pagesRisk Factors For Diabetes Mellitusavinash dhameriyaPas encore d'évaluation
- How To Reverse Diabetes MellitusDocument4 pagesHow To Reverse Diabetes MellitusAjay YadavPas encore d'évaluation
- DM ReportDocument16 pagesDM ReportWendy EscalantePas encore d'évaluation
- Blood Glucose (Homeostasis)Document17 pagesBlood Glucose (Homeostasis)areebaPas encore d'évaluation
- 11. Hypoglycemia and hyperglycemia GM eng 2022Document3 pages11. Hypoglycemia and hyperglycemia GM eng 2022kabulkabulovich5Pas encore d'évaluation
- Test 1 - Psio303aDocument28 pagesTest 1 - Psio303aErika Aranda0% (1)
- 3 Types of Organism As A ProductDocument2 pages3 Types of Organism As A ProductShaistha FriasPas encore d'évaluation
- Pathophysiology of Gestational DMDocument3 pagesPathophysiology of Gestational DMAnonymous GtR96jCPas encore d'évaluation
- Understanding Diabetes and Kidney DiseaseDocument11 pagesUnderstanding Diabetes and Kidney DiseasePhoespha MayangsariePas encore d'évaluation
- The Diabetes Bible: Diabetes and Pre-diabetes for beginners guide & education for effective self management, diet & nutrition, treatment solutions to symptoms & prevention, to improve quality of lifeD'EverandThe Diabetes Bible: Diabetes and Pre-diabetes for beginners guide & education for effective self management, diet & nutrition, treatment solutions to symptoms & prevention, to improve quality of lifePas encore d'évaluation
- Type 1 Diabetes Mellitus (Type 1 DM) : 1. Describe Patof Insulin PD DMDocument5 pagesType 1 Diabetes Mellitus (Type 1 DM) : 1. Describe Patof Insulin PD DMTheddyon BhenliePas encore d'évaluation
- 06.disorder of Carbohydrate MetabolismDocument47 pages06.disorder of Carbohydrate MetabolismRizka NizarPas encore d'évaluation
- Overview of Type IIDiabetesand Current ResearchDocument2 pagesOverview of Type IIDiabetesand Current ResearchkimberjinglePas encore d'évaluation
- 14 - Blood Glucose HomeostasisDocument34 pages14 - Blood Glucose HomeostasischeckmatePas encore d'évaluation
- Diabetic Ketoacidosis: From Wikipedia, The Free EncyclopediaDocument4 pagesDiabetic Ketoacidosis: From Wikipedia, The Free EncyclopediaTika Fajar WulandariPas encore d'évaluation
- Carbohydrates HandoutDocument15 pagesCarbohydrates HandoutnullPas encore d'évaluation
- Types & Causes of DiabetesDocument4 pagesTypes & Causes of DiabetesNor Ashikin IsmailPas encore d'évaluation
- Suarez - 1-Biochemistry of Glycogen and DiabetesDocument5 pagesSuarez - 1-Biochemistry of Glycogen and Diabetesjelyn suarezPas encore d'évaluation
- Hdp301diabetes 2021Document6 pagesHdp301diabetes 2021Linda NguyenPas encore d'évaluation
- Diabetes MellitusDocument32 pagesDiabetes MellitusGloria KikiPas encore d'évaluation
- Diabetes MellitusDocument1 pageDiabetes MellitusKenia GómezPas encore d'évaluation
- Physiology of Diabetes: Dr. Solomon Sathishkumar. MDDocument36 pagesPhysiology of Diabetes: Dr. Solomon Sathishkumar. MDKhaled LajmiPas encore d'évaluation
- A P Research Paper DiabetesDocument8 pagesA P Research Paper Diabetesapi-353042837Pas encore d'évaluation
- Glucose Homeostasis Disorders: by Julyn Marie Elnas Abad-GallardoDocument53 pagesGlucose Homeostasis Disorders: by Julyn Marie Elnas Abad-GallardoMarielle J GarciaPas encore d'évaluation
- 10 of The Most Costly Computer Viruses of All TimeDocument2 pages10 of The Most Costly Computer Viruses of All TimeEdwardPaulMadronio-NovedaPas encore d'évaluation
- EdwardDocument1 pageEdwardEdwardPaulMadronio-NovedaPas encore d'évaluation
- Head InjuryDocument17 pagesHead InjuryWilson MachadoPas encore d'évaluation
- Nursing Care Plan Peptic UlcerDocument3 pagesNursing Care Plan Peptic UlcerAntonio G. Cordillon100% (1)
- Edward Paul M. NovedaDocument27 pagesEdward Paul M. NovedaEdwardPaulMadronio-NovedaPas encore d'évaluation
- Weeks 1-2 Carb Cycling Blueprint FINAL r1Document24 pagesWeeks 1-2 Carb Cycling Blueprint FINAL r1Umashanker VishwakarmaPas encore d'évaluation
- Ketogenic DietDocument60 pagesKetogenic DietKanchi Mitra BhargavPas encore d'évaluation
- 20 Apple Cider Vinegar Uses + 6 Health Benefits Explained in DetailDocument17 pages20 Apple Cider Vinegar Uses + 6 Health Benefits Explained in DetailAnjar WijayadiPas encore d'évaluation
- Prediabetes A High Risk State For Diabetes Development PDFDocument12 pagesPrediabetes A High Risk State For Diabetes Development PDFDario Neri CortezPas encore d'évaluation
- Metabolic Syndrome: Causes and Risks of Cluster of 5 ConditionsDocument119 pagesMetabolic Syndrome: Causes and Risks of Cluster of 5 ConditionsMudassar SattarPas encore d'évaluation
- Seminar: Epidemiology and Global Trends in Type 2 DiabetesDocument18 pagesSeminar: Epidemiology and Global Trends in Type 2 DiabetesRoopaPas encore d'évaluation
- Reversing Diabetes in 21 Days PDFDocument311 pagesReversing Diabetes in 21 Days PDFAparna Ganapathi100% (5)
- Mock Exam 3Document42 pagesMock Exam 3Althea Karmylle M. BonitaPas encore d'évaluation
- Nutrients: Use of An Extract of Annona Muricata Linn To Prevent High-Fat Diet Induced Metabolic Disorders in C57BLDocument22 pagesNutrients: Use of An Extract of Annona Muricata Linn To Prevent High-Fat Diet Induced Metabolic Disorders in C57BLjenegnePas encore d'évaluation
- Mechanism and Effects of Glucose Absorption During An Oral Glucose Tolerance Test Among Females and MalesDocument12 pagesMechanism and Effects of Glucose Absorption During An Oral Glucose Tolerance Test Among Females and MalesdiskaPas encore d'évaluation
- 1-S2.0-S2352939316000026-Main Microbiomul Si Glicemia PDFDocument15 pages1-S2.0-S2352939316000026-Main Microbiomul Si Glicemia PDFLUTICHIEVICI NATALIAPas encore d'évaluation
- Ibarra 2023 Safety and Effectiveness of A Standardized IntravenousDocument7 pagesIbarra 2023 Safety and Effectiveness of A Standardized IntravenousEdward ElBuenoPas encore d'évaluation
- 10 Reasons Why Sugar Is Bad For YouDocument5 pages10 Reasons Why Sugar Is Bad For YouAgussPas encore d'évaluation
- Synergic Antiobesity Effects of Bitter Melon Water Extract and Platycodin-D in Genetically Obese MiceDocument9 pagesSynergic Antiobesity Effects of Bitter Melon Water Extract and Platycodin-D in Genetically Obese Micegege wpPas encore d'évaluation
- Case of DM Type 2 UTIDocument38 pagesCase of DM Type 2 UTIMichelePas encore d'évaluation
- Farmakologi Sindrom Metablik CitraDocument63 pagesFarmakologi Sindrom Metablik CitraAnonymous UaWvUXL6L1Pas encore d'évaluation
- Appes Abstracts: Oral PresentationsDocument146 pagesAppes Abstracts: Oral PresentationsstonePas encore d'évaluation
- 2005 ProactiveDocument11 pages2005 ProactiveBanky SupittayapornPas encore d'évaluation
- Pomegranate Health BenefitsDocument27 pagesPomegranate Health BenefitscommuniversityPas encore d'évaluation
- LaValle - Dietary SupplementsDocument122 pagesLaValle - Dietary Supplementsortizjesus18Pas encore d'évaluation
- Classification of Diabetes Mellitus - Endotext - NCBI BookshelfDocument11 pagesClassification of Diabetes Mellitus - Endotext - NCBI BookshelfSwati ParijaPas encore d'évaluation
- Diabetes Mellitus: Type 1 DM Type 2 DM Gestational DM Other TypesDocument20 pagesDiabetes Mellitus: Type 1 DM Type 2 DM Gestational DM Other Typesmohamed mowafeyPas encore d'évaluation
- Artemis P. Simopoulos, Francesco Visioli More On Mediterranean Diets World Review of Nutrition and Dietetics Vol 97 2006Document255 pagesArtemis P. Simopoulos, Francesco Visioli More On Mediterranean Diets World Review of Nutrition and Dietetics Vol 97 2006tazzycaPas encore d'évaluation
- MNT Case Study 2Document4 pagesMNT Case Study 2api-344809505100% (2)
- CH2009 Dim MakDocument50 pagesCH2009 Dim Makfedericoasensio100% (6)
- E-Book Vegan FitnessDocument92 pagesE-Book Vegan FitnessMarian Sava100% (4)
- Anik Widijanti: Clinical Pathology Department Saiful Anwar Hospital / Medical Faculty Brawijaya University MALANGDocument48 pagesAnik Widijanti: Clinical Pathology Department Saiful Anwar Hospital / Medical Faculty Brawijaya University MALANGTiti 9Pas encore d'évaluation
- Non-Alcoholic Fatty Liver DiseaseDocument35 pagesNon-Alcoholic Fatty Liver Diseaserigueiram100% (1)
- epid15,+10.+ARMYA+ZAKIAH ERNI+A 184-191Document8 pagesepid15,+10.+ARMYA+ZAKIAH ERNI+A 184-191Selmitha SariPas encore d'évaluation
- Comp - Appraisal Pre Final Assignment 2Document32 pagesComp - Appraisal Pre Final Assignment 2Joanne Bernadette AguilarPas encore d'évaluation