Académique Documents
Professionnel Documents
Culture Documents
Thermo
Transféré par
Melvin EsguerraDescription originale:
Titre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Thermo
Transféré par
Melvin EsguerraDroits d'auteur :
Formats disponibles
1. The normal body temperature of a healthy male is 98oF. What is the temperature in Celsius? 2.
Express a 10oC temperature difference in Kelvin, Rankine and Fahrenheit. 3. A Bourdon Gage and a mercury (=13,600 kg/m3) manometer are connected to a gas tank to measure its pressure. If the reading on the pressure gage is 85 kPa, determine h in mm.
4. A gas is contained in a vertical, frictionless piston cylinder device. The piston has a mass of 10 kg with a cross-sectional area of 20 cm2 and is pulled with a force of 100N. If the atmospheric pressure is 100 kPa, determine the pressure inside. Also determine the boundary work transfer, if the volume expands by 0.1 m3. 5. Determine what properties remain unchanged as the pressure of liquid water is increased from 100 kPa to 1 MPa (assume the temperature to be constant) 6. Determine the mass of air at 100 kPa, 25oC in a room with dimensions 5m x 5m x 5m. What-ifscenario: How would the conclusion change if the temperature, pressure and volume of the room were changed to new values? 7. The air in an automobile tire with a volume of 18 ft3 is at 90oF and 25 psig. Determine the amount of air to be added to bring the pressure up to 30 psig. Assume the atmospheric pressure to be 14.7 psia and the temperature and volume to remain constant. What-if-scenario: How would the conclusion change if the pressure goes up to 40 psig? 8. Calculate the change of internal energy as air is heated from 300K to 1000K using (a) perfect gas model, (b) ideal gas model. 9. Air at 15oC and 100 kPa enters the diffuser of a jet engine steadily with a velocity of 100 m/s. The inlet area is 0.2 m2. Determine the mass flow rate of the air. What-if-scenario: How would the conclusion change if the entrance velocity were 150 m/s? 10. Determine the specific volume of nitrogen gas at 10 MPa, 175K based on the generalized compressibility chart. What-if-scenario: How would the conclusion change if the working fluid were oxygen? 11. A 10 gallon tank contains 9 gallon liquid propane and the rest vapor at room temperature (30 oC). Calculate the pressure inside. What-if-scenario: How would the conclusion change if the room temperature were 15 oC? 12. Determine the amount of heat necessary to raise the temperature of 1 kg of aluminum from 30 oC to 100oC. What-if-scenario: How would the conclusion change if wood were heated instead of aluminum?
13. An insulated rigid tank contains 1.5 kg of helium at 30oC and 500 kPa. A paddle wheel with a power rating of 0.1 kW is operated within the tank for 30 minutes. Determine the final temperature and pressure. What-if-scenario: How would the conclusion change if the initial pressure were 100 kPa instead?
14. A person living in a 4m x 5m x 5m room turns on a 100-W fan before he leaves the warm room at 100 kPa, 30oC, hoping that the room will be cooler when he comes back after 5 hours. Disregarding any heat transfer determine the temperature he discovers when he comes back. What-if-scenario: How would the conclusion change if the fan power were 50 W instead? 15. An insulated rigid tank is divided into two equal parts by a membrane. At the beginning, one part contains 3 kg of Nitrogen at 500 kPa, 50oC, and the other part is completely evacuated. The membrane is punctured and the gas expands into the entire tank. Determine the final temperature and pressure. What-if-scenario: How would the conclusion change if the filled section contained 3 kg of Nitrogen at 300 kPa?
Vous aimerez peut-être aussi
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5795)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (895)
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (588)
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (345)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (121)
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (400)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (74)
- Aama 501.2-03Document8 pagesAama 501.2-03Gino TironiPas encore d'évaluation
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- Electrical Quick GuideDocument39 pagesElectrical Quick GuideMelvin EsguerraPas encore d'évaluation
- Planning and Layout of Ports and HarborsDocument1 pagePlanning and Layout of Ports and HarborsMelvin EsguerraPas encore d'évaluation
- An Example Problem On Wind Load Calculation According To NSCP 2010Document32 pagesAn Example Problem On Wind Load Calculation According To NSCP 2010Melvin Esguerra89% (9)
- Basic Instrumentation For Oil Gas Industries - Presentation - 29.08.10Document101 pagesBasic Instrumentation For Oil Gas Industries - Presentation - 29.08.10Tejas Patel100% (5)
- M.SC Industrial Chemistry: Internship ReportDocument44 pagesM.SC Industrial Chemistry: Internship ReportSahil BhuvaPas encore d'évaluation
- Astm F 431 - 03Document12 pagesAstm F 431 - 03Francisco GuerraPas encore d'évaluation
- Differential CalculusDocument1 pageDifferential CalculusMelvin EsguerraPas encore d'évaluation
- Chapter 1Document8 pagesChapter 1Melvin EsguerraPas encore d'évaluation
- XIV.-Judging Criteria and Scoring: Element Objective Scoring WeightDocument9 pagesXIV.-Judging Criteria and Scoring: Element Objective Scoring WeightMelvin EsguerraPas encore d'évaluation
- Integral Calculus Course Outline Prelim I. The Integration ProcessDocument1 pageIntegral Calculus Course Outline Prelim I. The Integration ProcessMelvin EsguerraPas encore d'évaluation
- The Integration Process: D FX FX DX D DXDocument2 pagesThe Integration Process: D FX FX DX D DXMelvin EsguerraPas encore d'évaluation
- Design of Isolated Footings of Rectangular From Using A New ModelDocument21 pagesDesign of Isolated Footings of Rectangular From Using A New Modelप्रभु नाथ सिंहPas encore d'évaluation
- Analysis of Isolated Footing Subjected To AF and High BM-ICJ-June 2014Document21 pagesAnalysis of Isolated Footing Subjected To AF and High BM-ICJ-June 2014varam1Pas encore d'évaluation
- Bridge DesignDocument1 pageBridge DesignMelvin EsguerraPas encore d'évaluation
- Calculate Earthquake Forces On Buildings and StructuresDocument5 pagesCalculate Earthquake Forces On Buildings and StructuresMelvin EsguerraPas encore d'évaluation
- 1.7.2. Layout of BreakwatersDocument2 pages1.7.2. Layout of BreakwatersMelvin EsguerraPas encore d'évaluation
- Rectangular Column CalculationsDocument1 pageRectangular Column CalculationsMelvin EsguerraPas encore d'évaluation
- 1.6. Sediment Transport and DredgingDocument1 page1.6. Sediment Transport and DredgingMelvin EsguerraPas encore d'évaluation
- Bearing Capacity QuizDocument1 pageBearing Capacity QuizMelvin EsguerraPas encore d'évaluation
- Highway 1Document6 pagesHighway 1Melvin EsguerraPas encore d'évaluation
- Architectural Engineering Technical Design Buildings Architect Structural Engineer Interior Designers ContractorsDocument1 pageArchitectural Engineering Technical Design Buildings Architect Structural Engineer Interior Designers ContractorsMelvin EsguerraPas encore d'évaluation
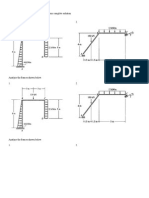
- Prelim Quiz!: Analyze The Frames Shown Below. Show Your Complete SolutionDocument2 pagesPrelim Quiz!: Analyze The Frames Shown Below. Show Your Complete SolutionMelvin EsguerraPas encore d'évaluation
- Planning and Layout of PortsDocument1 pagePlanning and Layout of PortsMelvin EsguerraPas encore d'évaluation
- Wind Load Calc InchfootcalcDocument9 pagesWind Load Calc InchfootcalcMelvin EsguerraPas encore d'évaluation
- Planning and Layout of PortsDocument2 pagesPlanning and Layout of PortsMelvin EsguerraPas encore d'évaluation
- Prefixes For GlobeDocument1 pagePrefixes For GlobeMelvin EsguerraPas encore d'évaluation
- How Do I View Multiple Excel Workbook Sheets Side by SideDocument1 pageHow Do I View Multiple Excel Workbook Sheets Side by SideMelvin EsguerraPas encore d'évaluation
- How Do I Create A New Duplicate Window in Excel?Document3 pagesHow Do I Create A New Duplicate Window in Excel?Melvin EsguerraPas encore d'évaluation
- Telecom Prefixes Prefixes For Globe/tmDocument1 pageTelecom Prefixes Prefixes For Globe/tmMelvin EsguerraPas encore d'évaluation
- Hiding The Formula by Protecting The Excel SheetDocument1 pageHiding The Formula by Protecting The Excel SheetMelvin EsguerraPas encore d'évaluation
- Prefixes For SmartDocument1 pagePrefixes For SmartMelvin EsguerraPas encore d'évaluation
- 0922 0923 0925 0932 0933 0942 New Prefix No. 0943 New Prefix No. NEXTEL Numbers 0979 - NEXTEL 0978 - NEXTELDocument1 page0922 0923 0925 0932 0933 0942 New Prefix No. 0943 New Prefix No. NEXTEL Numbers 0979 - NEXTEL 0978 - NEXTELMelvin EsguerraPas encore d'évaluation
- Model: BSF28Z.12L 210602265 / 670201025 Machine Card 01/22/2007 1 of 6Document173 pagesModel: BSF28Z.12L 210602265 / 670201025 Machine Card 01/22/2007 1 of 6Alonso MtZ100% (1)
- Dossat Principles of RefrigerationDocument554 pagesDossat Principles of RefrigerationJunaid Ameer100% (8)
- Pressure Transmitter For General Applications Model S-10, Standard Version Model S-11, Flush DiaphragmDocument4 pagesPressure Transmitter For General Applications Model S-10, Standard Version Model S-11, Flush Diaphragminfo5280Pas encore d'évaluation
- 2021 - Price List 1.0 - 12 01 2020Document4 pages2021 - Price List 1.0 - 12 01 2020Rifki AndriyanPas encore d'évaluation
- 6A. Koporetz Tom 2-Instrumentation Process Control Custom ScreenDocument44 pages6A. Koporetz Tom 2-Instrumentation Process Control Custom ScreenRajesh RoyPas encore d'évaluation
- Emi 2018Document72 pagesEmi 2018Pushpendra Pratap Singh0% (1)
- Module IDocument16 pagesModule IVenkata DineshPas encore d'évaluation
- VODOR压力表资料 PDFDocument2 pagesVODOR压力表资料 PDFliu zhao liu zhaoPas encore d'évaluation
- UKAS Calibration IntervalDocument18 pagesUKAS Calibration IntervalRudolf100% (1)
- IN20640701 MESSKO Product Catalogue enDocument28 pagesIN20640701 MESSKO Product Catalogue enchennupati99Pas encore d'évaluation
- Fluid Mechanics and Machinery Unit 2 Fluid Kinematics & DynamicsDocument40 pagesFluid Mechanics and Machinery Unit 2 Fluid Kinematics & DynamicsPalaniVelRajanPas encore d'évaluation
- EHP Guidelines District Heating SubstationsDocument72 pagesEHP Guidelines District Heating SubstationsMario TirabassiPas encore d'évaluation
- Solenoid ValveDocument34 pagesSolenoid ValveJusthyTaquiriPas encore d'évaluation
- Fundamentals of Vacuum Science-L02aDocument32 pagesFundamentals of Vacuum Science-L02aTonie Yanto Fanggi MbuikPas encore d'évaluation
- Fluid Mechanics Student NotebookDocument100 pagesFluid Mechanics Student Notebookrbabhi100% (1)
- CE 706 - Experiment-01Document5 pagesCE 706 - Experiment-01Alex SandroPas encore d'évaluation
- SPECIFICATIONDocument2 pagesSPECIFICATIONRahul RajPas encore d'évaluation
- Industrial Regulators Application Guide VI (TECHNICAL)Document136 pagesIndustrial Regulators Application Guide VI (TECHNICAL)steeven siregarPas encore d'évaluation
- 3D - DPG-6600 - DatasheetDocument2 pages3D - DPG-6600 - DatasheetJames GeorgePas encore d'évaluation
- Thermo HW SolutionsDocument35 pagesThermo HW SolutionsekantikdevoteePas encore d'évaluation
- CIE IGCSE Physics (0625) Pressure Notes PDF Pressure Pressure Measurement 11Document1 pageCIE IGCSE Physics (0625) Pressure Notes PDF Pressure Pressure Measurement 11Sarah KhanPas encore d'évaluation
- 2186Document144 pages2186Mohamed ShafeyPas encore d'évaluation
- HW 1 - MAE 3310 - 001 - Fall 2020-2Document3 pagesHW 1 - MAE 3310 - 001 - Fall 2020-2Belgium WafflesPas encore d'évaluation
- Metrology Lab Viva Voce QuestionsDocument6 pagesMetrology Lab Viva Voce Questionsmrbalaji88Pas encore d'évaluation
- Flat Jack DRCDocument4 pagesFlat Jack DRCpavan6595Pas encore d'évaluation