Académique Documents
Professionnel Documents
Culture Documents
221 08
Transféré par
a..all1Description originale:
Titre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
221 08
Transféré par
a..all1Droits d'auteur :
Formats disponibles
146
Liquid-Liquid Extraction (LLX)
Extraction is a liquid-liquid operation. It is a process of
transferring a solute from one
liquid phase to another immiscible
or partially miscible liquid in
contact with the first. The two
phases are chemically quite
different, which leads to a
separation of the components
according to their distribution or
partition between the two phases,
normally one organic and one
water. This is different from
distillation, in which the liquid is
partially vaporized to create
another (vapor) phase, but the two
phases are similar chemically.
147
Equilibrium Relations in Extraction
1. Phase rule.
In a liquid-liquid system, the minimum number of
components is three and we have two phases in
equilibrium. For a ternary system, the number of degrees
of freedom is three, as calculated by the phase rule (F = C
- P + 2 = 3 - 2 + 2 = 3). We have six variables:
temperature, pressure, and four concentrations. If the
pressure and temperature are specified, then setting one
concentration will fix the system. The other three
concentrations must be calculated from the phase
equilibrium.
2. Triangular coordinates and equilibrium data.
Since we have three components, the equilibrium data are
often expressed in equilateral triangular coordinates. This
is shown in the following figure.
Figure 1. Coordinates for a triangular diagram.
148
The three corners represent three pure components, A, B
and C. The point M is a mixture. The perpendicular
distance from the point M to any base line is the mass
fraction of the component at the corner opposite to that
base line. For example, the distance from M to base AB
is the mass fraction of C (x
C
= 0.40).
A com
Fig 2
misci
The tw
envel
separ
equili
also p
which
one p
Figur
The
curve
exper
and S
we c
mixtu
point
mixtu
equili
and Q
mmon p
, where
ble, an
wo pha
lope. A
ate into
ibrium
plotted
h is cal
phase re
re 2. Li
co
equili
e is
riments
S are
can ch
ure liqu
D in
ure is s
ibrium
Q.
phase d
e a pai
nd liqu
ase regi
An ori
o two c
tie line
d. The
lled the
egion.
iquid-li
ompone
ibrium
obtaine
s. If com
partiall
hoose
uid of B
n Fig.
separat
liquid
diagram
ir of co
id C d
ion is i
iginal m
conjuga
e throu
e two p
e Plait
iquid p
ents A a
solu
ed thr
mponen
ly mis
any b
B and S
3.
ed into
d phase
149
m is Typ
ompone
dissolve
include
mixture
ate pha
ugh poi
phases
point.
phase di
and B a
ubility
rough
nts B
cible,
binary
S, say
This
o two
es, P
Fig.3
ype I sy
ents A
es com
d insid
e of co
ases a a
int M.
are id
Outsi
iagram
are par
. Exper
ystem a
and B
mpletely
de below
omposi
and b w
Other
dentical
ide the
m where
rtially m
rimenta
and sho
are par
y in A
w the c
ition M
which a
tie line
l at po
envelo
e
miscible
al solub
wn in
rtially
or B.
curved
M will
are on
es are
int P,
ope is
e.
bility.
150
Under constant temperature, if we add component A into
this binary mixture drop by drop, the composition of the
ternary mixture will change along line DA. The ratio of
B/S is constant while the amount of A is changing. When
the amount of A is just to make the mixture from two
phases to one single homogeneous phase, the composition
is represented by the point D. By repeating this
procedure for other binary mixtures with compositions of
E, F, G, we obtain the points E, F, G. The curve links
the points PDEFGQ is the equilibrium solubility curve.
If B and S are completely immiscible, the two end points
P and Q on the solubility curve will superimpose to the
top points B and S, respectively.
Conjugate line. As far as some equilibrium tie lines are
available, other tie lines can be obtained by interpolation
with the help of conjugate curve. Assuming the tie lines
E
1
R
1
, E
2
R
2
, E
3
R
3
, E
4
R
4
are known, we draw a vertical line
from E
1
, which
intersects the
horizontal line from
R
1
at point F.
Similary, the vertical
lines from E
2
, E
3
, E
4
,
intersects the
horizontal lines from
R
2
, R
3
, R
4
at points G,
H and J. The curve
joining FGHJ and the
plait point P is called
the conjugate line.
151
Example E1: Ternary acetic acid-benzene-water mixture.
The liquid-liquid equilibrium data at 25
o
C is given in the
following table. In the right-angled triangle, illustrate
(1) Solubility curve
(2) Tie lines for experiments of Nos. 2, 3, 4, 6, 8
(3) Plait point and conjugate line
No.
Benzene phase (mass %) Water phase (mass %)
acetic
acid
benzene water acetic
acid
benzene water
1 0.15 99.85 0.001 4.56 0.04 95.4
2 1.4 98.56 0.04 17.7 0.20 82.1
3 3.27 96.62 0.11 29.0 0.40 70.6
4 13.3 86.3 0.4 56.9 3.3 39.8
5 15.0 84.5 0.5 59.2 4.0 36.8
6 19.9 79.4 0.7 63.9 6.5 29.6
7 22.8 76.35 0.85 64.8 7.7 27.5
8 31.0 67.1 1.9 65.8 18.1 16.1
9 35.3 62.2 2.5 64.5 21.1 14.4
10 37.8 59.2 3.0 63.4 23.4 13.2
11 44.7 50.7 4.6 59.3 30.0 10.7
12 52.3 40.5 7.2 52.3 40.5 7.2
(1)The data points are plotted in the right-angled triangle
coordinates, joining the points gives the solubility curve.
(2) The experiments of Nos. 2, 3, 4, 6, 8 are shown as
points R
1
, E
1
, R
2
, E
2
, R
3
, E
3
, R
4
, E
4
, R
5
, E
5
. The tie lines
are the straight lines R
1
E
1
, R
2
E
2
, R
3
E
3
, R
4
E
4
, R
5
E
5
.
152
(3) The last set of data have the same composition in the
two phases, which is the plait point. The auxiliary curve
is obtained by drawing vertical lines from E
1
, E
2
, E
3
, E
4
,
E
5
, which intersect the horizontal lines from R
1
, R
2
, R
3
,
R
4
, R
5
at points G, H, I, J, L. Joining GHIJLP gives the
conjugate line.
153
Example E2: For the ternary system of example E1 at
25
o
C, a mixture is separated into two liquid phases after
settling. One phase contains 15% acetic acid, 0.5% water
and the rest being benzene (all mass %). Use the
conjugate line in example E1 to determine the
composition of the other conjugate liquid phase and draw
the tie line.
Solution: The solubility and conjugate curves are given.
1. Find the composition of 15% acetic acid, 0.5% water
as point R.
2. Draw a horizontal line from R to intersect the
conjugate line at Q.
3. Draw a vertical line from Q to intersect the
solubility curve at E, which is the composition of the
other conjugate phase, 59% acetic acid, 37% water,
4% benzene.
4. Join RE to obtain the tie line.
154
3. Equilibrium data on rectangular coordinates.
Because of the special coordinates, the triangular diagram
is not convenient. The liquid-liquid phase equilibrium is
more often presented in rectangular coordinates, which is
shown in Fig. 4 for acetic acid (A) - water (B) - isopropyl
ether solvent (C).
Figure 4. Acetic acid (A)-Water (B)-Isopropyl ether (C)
liquid-liquid phase diagram at 293 K (20
o
C).
The solvent pair B and C are partially miscible. The
concentration of A is plotted on the horizontal axis and
that of C on the vertical axis. The concentration of B is
calculated from the following equation
155
x x x
B A C
= 10 . y y y
B A C
= 10 .
A tie line gi is shown connecting the water-rich layer i,
called the raffinate layer, and the ether-rich solvent layer
g, called the extract layer. The raffinate composition is
designated by x, and the extract by y. Hence, the mass
fraction of C is designated as y
C
in the extract laywer and
as x
C
in the raffinate layer. To construct the tie line gi
using the equilibrium y
A
-x
A
plot below the phase diagram,
vertical lines to g and i are drawn.
Example E3: Material balance for equilibrium layers
An original mixture weighing 100 kg and containing 30
kg of isopropyl ether (C), 10 kg of acetic acid (A), and 60
kg water (B) is equilibrated and the equilibrium phases
separated. What are the compositions of the two
equilibrium phases.
Solution: The composition of the original mixture is
x
C
= 0.30, x
A
= 0.10, x
B
= 0.60
This composition is plotted as point h on Fig. 4. The tie
line gi is obtained through point h by trial and error. The
composition of the extract (ether) layer at g is y
A
= 0.04,
y
C
= 0.94, and y
B
= 1 0.04 0.94 = 0.02 mass fraction.
The raffinate (water) layer composition at i is x
A
= 0.12,
x
C
= 0.02, and x
B
= 1 0.12 0.02 = 0.86 mass fraction.
156
In the above system (Fig. 4) the solvent pair B and C are
partially miscible, while A is totally soluble in B or C. It
is also common for some other systems that both pairs, A
and C, and B and C are partially miscible. This is Type II
system and shown in figure 5.
Figure 5. Liquid-liquid phase diagram where the
solvent pairs A-C and B-C are partially miscible.
Examples are the systems of Styrene (A) -Ethylbene (B) -
Diethylene Glycol (C), and Chlorobenzene (A) -
Methylethyl Ketone (B) -Water (C).
157
Single-Stage Equilibrium Extraction
In an extraction process we have two entering streams (L
kg and V kg) which are NOT in equilibrium, as shown in
Fig. 6. The solvent, as stream V
2
, enters and the stream
L
0
enters from the other side. The two entering streams
are mixed and equilibrated and then exit as streams L
1
and V
1
, which are in equilibrium with each other. To find
the final product compositions in the two phases, it is
required to know the mixture total mass and composition
(point M). This can be obtained by material balances.
After the point M is identified, the product composition
can be found by the equilibrium tie line.
158
Figure 6. Single-stage Liquid-liquid extraction:
(a) process flow diagram, (b) phase diagram.
Material balances:
Overall: L V L V M
0 2 1 1
+ = + = (1)
(A): L x V y L x V y Mx
A A A A AM 0 0 2 2 1 1 1 1
+ = + = (2)
(C): L x V y L x Vy Mx
C C C C CM 0 0 2 2 1 1 1 1
+ = + = (3)
Since x
A
+ x
B
+ x
C
= 1 , an equation for B is not needed.
because L
0
and V
2
are known, values of M, x
AM
, and x
CM
,
can be found from Eqs. (1) to (3). L
1
and V
1
are obtained
by drawing a tie line through point M.
159
Derivation of lever-arm rule for graphical addition.
In Figure 7 we have two streams (L & V) mixed to give a
resulting mixture stream M kg total mass.
((b)
(b)
Figure 7. Graphical addition and lever-arm rule:
(a) process flow, (b) graphical addition.
By doing material balances, we have
Overall: V L M + = (4)
(A): Lx Vy Mx
A A AM
+ == (5)
(C): Lx Vy Mx
C C CM
+ == (6)
Combining Eqs. (4) & (5), and (4) & (6), we have
L
V
x y
x x
AM A
A AM
=
(7)
L
V
x y
x x
CM C
C CM
=
(8)
Equating Eqs. (7) & (8) and rearranging,
x x
x x
x y
x y
C CM
A AM
CM C
AM A
(9)
The left side is the slope of line LM and the right side is
the slope of line MV. Because the two slopes are the
same and the two lines have a common point M, the three
160
points L, M, and V must be on a straight line. The lever-
arm rule is
L
V
VM
LM
= &
L
M
VM
LV
= (10)
Example E4: Amount of phases in solvent extraction
The compositions of the two equilibrium layers in
example E1 are:
for the extract layer (V),
y
A
= 0.04, y
B
= 0.02, y
C
= 0.94
for the raffinate layer (L),
x
A
= 0.12, x
B
= 0.86, x
C
= 0.02
The original mixture contained 100 kg and x
AM
= 0.10.
Determine the amounts of V and L.
Solution: The overall material balance is
V + L = M = 100 kg
The material balance of A is
V(0.04) + L(0.12) = 100(0.10)
Hence,
V = 75 kg, L = 25 kg
Alternatively, using the lever-arm rule, the distance hg in
Fig. 4 is measured as 4.2 units and gi as 5.8 units. Then
L
N
=
L
1uu
=
hg
g
=
4.2
S.8
Solving, L = 72.5 kg and V = 27.5 kg, which is in
reasonable close agreement with the material balance
method.
161
Countercurrent Multistage Extraction
162
1. Countercurrent process and overall balances
A countercurrent multistage process is shown in Fig. 8.
Figure 8. Countercurrent multistage extraction process
flow diagram
The overall balance on all N stages is
L V L V M
N N 0 1 1
+ = + =
+
(11)
where M is the total mass (kg/h) and is a constant, L
0
the
inlet feed flow rate (kg/h), V
N+1
the inlet solvent flow rate
(kg/h), V
1
the exit extract stream, and L
N
the exit raffinate
stream. Material balance on C gives
L x V y L x V y Mx
C N C N N C N C CM 0 0 1 1 1 1
+ = + =
+ + , ,
(12)
x
CM
is obtained By solving Eqs. (11) & (12)
x
L x V y
L V
L x V y
L V
CM
C N C N
N
N CN C
N
=
+
+
=
+
+
+ +
+
0 0 1 1
0 1
1 1
1
,
(13)
A similar balance on component A gives
x
L x V y
L V
L x V y
L V
AM
A N A N
N
N AN A
N
=
+
+
=
+
+
+ +
+
0 0 1 1
0 1
1 1
1
,
(14)
So the point M, which ties together the two entering
streams (usually known) and the two exit streams, can be
located. The desired exit composition x
AN
is often set,
which is on the equilibrium curve (phase boundary). Then
the line L
N
M is extended to intersect the phase boundary
of the extract phase to give V
1
composition.
163
Example E5: Pure solvent isopropyl ether (C) at the rate
of V
N+1
= 600 kg/h is being used to extract an aqueous
solution of L
0
= 200 kg/h containing 30 wt % acetic acid
(A) and 70 wt % water (B) by countercurrent multistage
extraction. The desired exit acetic acid concentration in
the aqueous phase is 4%. Calculate the compositions and
amounts of the ether extract V
1
and the aqueous raffinate
L
N
. The equilibrium data at 20
o
C, 1 atm, are given and
plotted below.
Water phase
(mass fraction)
isopropyl ether phase
(mass fraction)
acetic
acid
(x
A
)
Water
(x
B
)
isopropyl
ether (x
C
)
acetic
acid
(y
A
)
Water
(y
B
)
isopropyl
ether
(y
C
)
6.9e-3 0.9810 0.0120 1.8e-3 5.0e-3 0.9930
0.0141 0.9710 0.0150 3.7e-3 7.0e-3 0.9890
0.0289 0.9550 0.0160 7.9e-3 8.0e-3 0.9840
0.0642 0.9170 0.0190 0.0193 0.0100 0.9710
0.1330 0.8440 0.0230 0.0482 0.0190 0.9330
0.2550 0.7110 0.0340 0.1140 0.0390 0.8470
0.3670 0.5890 0.0440 0.2160 0.0690 0.7150
0.4430 0.4510 0.1060 0.3110 0.1080 0.5810
0.4640 0.3710 0.1650 0.3620 0.1510 0.4870
164
Solution:
The given values are
Pure solvent inlet:
V
N+1
= 600, y
A,N+1
= y
B,N+1
= 0, y
C,N+1
= 1,
Feed:
L
0
= 200, x
A0
= 0.3, x
B0
= 0.7, x
C0
= 0,
Raffinate:
x
AN
= 0.04.
V
N+1
and L
0
are located by the compositions.
Since L
N
is on the phase boundary of the raffinate phase,
it can be plotted at x
AN
= 0.04 & we find x
CN
= 0.017.
165
The composition of the mixture, x
CM
and x
AM
, are
calculated by Eqs. (13) & (14) as 0.75 & 0.075 and used
to plot point M. V
1
is located by drawing a line from L
N
through M & extending it until it intersects the phase
boundary in the extract phase. This gives y
A1
= 0.08 &
y
C1
= 0.90.
By solving Eqs. (11) & (12), L
N
= 136 kg/h & V
1
= 664
kg/h.
2.Stage-to-stage calculation for countercurrent extraction
The next step is to go stage by stage to determine the
concentrations at each stage and the total number of
stages N needed to reach L
N
in the process.
Making a total balance on stage 1 and then on stage n,
L V L V
0 2 1 1
+ = + (15)
L V L V
n n n n +
+ = +
1 1
(16)
The above equations can be rearranged as
L V L V L V L V
n n N N 0 1 1 2 1 1
= = = = =
+ +
...
(17)
The value of is constant for all stages. The coordinates
of the operating point can be obtained by material
balances on A, B or C:
166
L x V y L x V y x
A A N AN N A N A 0 0 1 1 1 1
= = =
+ +
...
,
(18)
L x V y L x V y x
C C N CN N C N C 0 0 1 1 1 1
= = =
+ +
...
,
(19)
x
L x V y
L V
L x V y
L V
A
A A
N AN N A N
N N
+ +
+
0 0 1 1
0 1
1 1
1
,
(20)
Similar Eqs. for x
B
& x
C
can be obtained. This point
is located either by its coordinates as calculated by Eq.
(20) or graphically as the intersection of lines L
0
V
1
and
L
N
V
N+1
. The method to locate V
1
has been discussed in
example E5. All the operating lines (L
0
V
1
, L
1
V
2
,
L
n
V
n+1
, ... , L
N
V
N+1
) must pass through the common point
.
To graphically determine the number of stages, follow the
procedures below.
(1) locate L
0
, V
N+1
and L
N
by their compositions.
(2) draw a line L
0
V
N+1
, and locate the mixture point M
by Eq. (13) or (14).
(3) draw a line from L
N
through M & extend it until it
intersects the phase boundary, where is V
1
.
(3) extend lines L
0
V
1
, and L
N
V
N+1
, which will intersect
at the common operating point .
(4) start at L
0
and draw a line L
0
which intersects the
phase boundary at V
1
.
(5) draw an equilibrium tie line through V
1
to locate L
1
.
(6) draw a line L
1
to give V
2
at the phase boundary.
(7) a tie line from V
2
gives L
2
. This is continued until
the desired L
N
is reached.
167
Alternately, the point can firstly be located using Eq.
(20). Then we start at L
0
and draw a line L
0
to locate V
1
.
Then an equilibrium tie line through V
1
locates L
1
. Line
L
1
is drawn to give V
2
. A tie line from V
2
gives L
2
.
This is continued until the desired L
N
is reached.
168
Example E6: Number of stages in countercurrent extraction
Pure isopropyl ether (C) of 450 kg/h is being used to
extract an aqueous solution of 150 kg/h with 30 wt %
acetic acid (A) and 70 wt % water (B) by countercurrent
multistage extraction. The exit acid concentration in the
aqueous phase is 10 wt %. Calculate the number of
stages required.
Solution: draw a diagram,
Known values:
Pure solvent from N+1:
V
N+1
= 450 kg/h
y
A,N+1
= y
B,N+1
= 0
y
C,N+1
= 1.0,
Feed:
L
0
= 150 kg/h
x
A0
= 0.3
x
B0
= 0.7
x
C0
= 0
Exit in water phase:
x
AN
= 0.1.
L
N
must be in the raffinate solubility line.
The points V
N+1
, L
0
, and L
N
are plotted.
169
x
A
, y
A
0.0 0.1 0.2 0.3 0.4 0.5
x
C
,
y
C
0.0
0.2
0.4
0.6
0.8
1.0
A (acetic acid)
B
(water)
C (isopropyl ether)
L
0
L
N
V
1
V
N+1
The mixture points are found by Eqs. (13) & (14), x
CM
=
0.75, x
AM
= 0.075. The point V
1
is located as the
intersection of line L
N
M with the phase boundary in the
extract phase, y
A1
= 0.072, y
C1
= 0.895.
170
Then lines L
0
V
1
, and L
N
V
N+1
is drawn to locate the point
.
x
A
, y
A
-0.1 0.0 0.1 0.2 0.3 0.4 0.5
x
C
,
y
C
0.0
0.2
0.4
0.6
0.8
1.0
1.2
1.4
A (acetic acid)
B
(water)
C (isopropyl ether)
L
0
L
N
V
1
V
N+1
171
Starting at L
0
we draw a line L
0
to locate V
1
. Then an
equilibrium tie line through V
1
locates L
1
. Line L
1
is
drawn to give V
2
. A tie line from V
2
gives L
2
. A final tie
line gives L
3
, which is beyond the desired L
N
. Hence,
about 2.5 theoretical stages are needed.
172
3. McCabe-Thiele method
Stepping off many stages on a triangular diagram can be
difficult and inaccurate. More accurate calculations can
be done with a McCabe-Thiele diagram. Here we focus
on the concentration of solute in the extract and raffinate
phases. The diagram does not show the concentration of
the diluents in the extract or the concentration of solvent
in the raffinate. These minor components of both phases
are accounted for in determining the total flow of extract
and raffinate, which affects the position of the operating
line.
In the McCabe-Thiele diagram, the equilibrium data are
shown on a rectangular graph, where the mass fraction of
solute in the extract (V) phase, y
A
, is plotted as the
ordinate and the mass fraction of solute in the raffinate (L)
phase, x
A
, as the abscissa. The conversion of equilibrium
tie line in the triangle diagram to the y-x digram is shown
below.
173
174
Since the total flow rates are not constant, the triangular
diagram and the point are used to plot a curved
operating line on the McCabe-Thiele diagram. This
construction is illustrated in the following figure for a
single point.
The two end points of the operating line are already given
in example E6. (x
AN
= 0.1, y
A,N+1
= 0), and (x
A0
= 0.3, y
A1
= 0.072).
For any arbitrary operating line (must go through ), the
values of the extract and raffinate concentrations of A are
determined from the phase diagram using the common
point and transferred to the y-x diagram, as shown in
the following figure.
The number of stages is then calculated by stepping off
the triangles with the operating and equilibrium lines,
which is about 2 in this case.
175
176
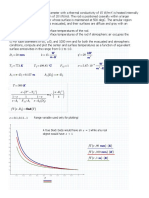
4. Minimum solvent rate
If a solvent rate V
N+1
is selected at too low a value, a
limiting case will be reached with an operating line
through and a tie line being the same. Then an infinite
number of stages will be needed to reach the desired
separation. The minimum amount of solvent is reached.
For actual operation a greater amount of solvent must be
used.
The procedure to obtain
this minimum solvent rate
is as follows and shown in
the right figure. Firstly
line L
N
V
N+1
is extended,
then all tie lines between
L
0
and L
N
are drawn to
intersect the extended line
L
N
V
N+1
. The intersection
farthest from V
N+1
(if is
in the L
N
side, which is the
case in the figure) or
nearest V
N+1
(if is on
the V
N+1
side) is the
min
point for minimum solvent.
The actual position of
must be farther from V
N+1
(if on the L
N
side) or nearer to V
N+1
(if on the V
N+1
side)
for a finite number of stages. The larger the amount of
solvent, the fewer the number of stages.
Minimum solvent for
countercurrent extraction.
177
This is proved as below.
If is in the L
N
side,
L
N
- V
N+1
=
L
N
= V
N+1
+
According to lever arms
rule:
(v
N+1
)v
N+1
L
N
= L
N
=( L
N
- V
N+1
) L
N
= L
N
L
N
- V
N+1
L
N
v
N+1
=
L
N
L
N
v
N+1
L
N
+L
N
=
L
N
V
N+1
L
N
AL
N
+1
Since L
N
and v
N+1
L
N
are constant, V
N+1
will achieve its
maximum when the length of L
N
is at its maximum.
178
If is in the V
N+1
side,
(v
N+1
)v
N+1
= L
N
L
N
v
N+1
=
L
N
L
N
v
N+1
= L
N
L
N
v
N+1
+v
N+1
v
N+1
= L
N
(1 +
L
N
V
N+1
V
N+1
A
)
Since L
N
and v
N+1
L
N
are constant, V
N+1
will achieve its
maximum when the length of v
N+1
is at its minimum.
Vous aimerez peut-être aussi
- 005251Document3 pages005251a..all1Pas encore d'évaluation
- Homogeneous LiquidDocument1 pageHomogeneous Liquida..all1Pas encore d'évaluation
- P&ID DiagramDocument6 pagesP&ID DiagramMejdi Sylas ToudjiPas encore d'évaluation
- TessDocument6 pagesTessa..all1Pas encore d'évaluation
- TessDocument6 pagesTessa..all1Pas encore d'évaluation
- TessDocument6 pagesTessa..all1Pas encore d'évaluation
- TessDocument6 pagesTessa..all1Pas encore d'évaluation
- Ac102 ch7Document22 pagesAc102 ch7Mohammed OsmanPas encore d'évaluation
- Handout 3Document6 pagesHandout 3a..all1Pas encore d'évaluation
- Liquid Liquid ExtractionDocument40 pagesLiquid Liquid ExtractionMohsin Ehsan100% (1)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5783)
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (890)
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (399)
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (265)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (72)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (344)
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (119)
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)
- DYNROT: A Matlab Toolbox For Rotordynamics Analysis: January 1994Document27 pagesDYNROT: A Matlab Toolbox For Rotordynamics Analysis: January 1994saurabhchandrakerPas encore d'évaluation
- Said DissertationDocument129 pagesSaid DissertationSaeed AzarPas encore d'évaluation
- Technorama Hausprospekt enDocument42 pagesTechnorama Hausprospekt enSriheri DeshpandePas encore d'évaluation
- Chemical Solution Density & ViscosityDocument18 pagesChemical Solution Density & ViscosityLee JianPas encore d'évaluation
- Gauge Mechanics-SardanashvilyDocument358 pagesGauge Mechanics-SardanashvilyJazon Bryan100% (1)
- 1603 Physics Paper With Ans Sol EveningDocument8 pages1603 Physics Paper With Ans Sol EveningRahul RaiPas encore d'évaluation
- EE305 Lecture 2 Instrument TypesDocument22 pagesEE305 Lecture 2 Instrument TypesFrank WhitePas encore d'évaluation
- Sample Problems 6717 PDFDocument10 pagesSample Problems 6717 PDFnagham radiPas encore d'évaluation
- Basic Electrical Engineering: BY R. Sivaprasad, Lecturer in Eee, Govt. Polytechnic, SatyaveduDocument77 pagesBasic Electrical Engineering: BY R. Sivaprasad, Lecturer in Eee, Govt. Polytechnic, Satyavedurathina4careerPas encore d'évaluation
- Buoyancy 100806114902 Phpapp02Document11 pagesBuoyancy 100806114902 Phpapp02saryPas encore d'évaluation
- MSL Technical Guide 18 Resistance Measurement For ThermometryDocument5 pagesMSL Technical Guide 18 Resistance Measurement For ThermometrydlonguinhoPas encore d'évaluation
- HW 3Document2 pagesHW 3Purusharth SemwalPas encore d'évaluation
- Basic Electronics 2Document19 pagesBasic Electronics 2Miss WorldPas encore d'évaluation
- Chemical KineticsDocument37 pagesChemical KineticsM H Alif HossainPas encore d'évaluation
- Thesis W QuakDocument178 pagesThesis W Quakdr_kh_ahmedPas encore d'évaluation
- Notes LT3Document16 pagesNotes LT3osmanfıratPas encore d'évaluation
- Lectures PDFDocument137 pagesLectures PDFFrancis Clinton Prashanth100% (1)
- Modeling and Analysis of Soil-Pile InteractionDocument21 pagesModeling and Analysis of Soil-Pile InteractionLhester NavascaPas encore d'évaluation
- Structure & Bonding - IONIC BONDINGDocument23 pagesStructure & Bonding - IONIC BONDINGTrishana GreenPas encore d'évaluation
- Thermal Physics - Rod in CylinderDocument5 pagesThermal Physics - Rod in CylinderLUIS HUMBERTO MARTINEZ PALMETHPas encore d'évaluation
- Axially Loaded Members PDFDocument50 pagesAxially Loaded Members PDFCharbel Saad SaadPas encore d'évaluation
- Lelm 316Document20 pagesLelm 316neerajPas encore d'évaluation
- Seminar Lesson PlanDocument18 pagesSeminar Lesson PlanNo one KnowsPas encore d'évaluation
- Siemens Vacumm Contactor 3tl6Document15 pagesSiemens Vacumm Contactor 3tl6Yong Ee VonnPas encore d'évaluation
- Solidworks Flow Simulation Project Report: (Company Logo Here)Document9 pagesSolidworks Flow Simulation Project Report: (Company Logo Here)zarzosa rabanalPas encore d'évaluation
- ORS SystemDocument4 pagesORS SystemlaxmanPas encore d'évaluation
- Bharathidasan University, Tiruchirappalli - 620 024. B.Sc. Physics Course Structure Under CBCSDocument27 pagesBharathidasan University, Tiruchirappalli - 620 024. B.Sc. Physics Course Structure Under CBCSSarjithPas encore d'évaluation
- Relativistic precession of planetary orbitsDocument5 pagesRelativistic precession of planetary orbitsdavePas encore d'évaluation
- Lateral Earth PressureDocument122 pagesLateral Earth PressureEric DelunaPas encore d'évaluation