Académique Documents
Professionnel Documents
Culture Documents
Inter Metallic S
Transféré par
محمد تانزيم ابراهيمDescription originale:
Titre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Inter Metallic S
Transféré par
محمد تانزيم ابراهيمDroits d'auteur :
Formats disponibles
11/7/2012
J.Muhammad Thanzeem Ibrahim M.Tech Manufacturing
Scope of the Presentation:
Definition Properties Classification Production Application Advantages Drawbacks
11/7/2012
J.Muhammad Thanzeem Ibrahim M.Tech Manufacturing
Definition
When a solution containing alloys of metals which have a limited
mutual solubility, solidifies it form a new phase at certain ratios.
This new phase possess crystal structures different from either
component and are called intermetallic compounds (sometimes abbreviated as IMCS)
Intermetallic formation between copper and tin
11/7/2012
J.Muhammad Thanzeem Ibrahim M.Tech Manufacturing
Properties of intermetallic compounds
Brittleness
Brittleness of intermetallic increase with decreasing lattice symmetry
and increasing unit cell size
Brittleness depends on
crystal symmetry specific characteristics of the particular phasesing unit cell size
Dislocations need high mobility and low energy, that imply the
existence of dislocation slip systems as twinning systems
11/7/2012
J.Muhammad Thanzeem Ibrahim M.Tech Manufacturing
Fracture initiation through the de-bonding of brittle particulates is
showed in the model below. The particles behave as stress concentrators as the ductility of the matrix is increased; localized plastic instability is the cause of this effect.
11/7/2012
J.Muhammad Thanzeem Ibrahim M.Tech Manufacturing
Brittle-to-Ductile Transition Temperature
At BDTT material typically exhibit a drastic increase of the fracture
toughness within a relatively narrow temperature range. As show on the graphic below (fig.2), the fracture toughness increase after BDTT so that the material can be considered as ductile
where J critical -Fracture toughness measurements
11/7/2012
J.Muhammad Thanzeem Ibrahim M.Tech Manufacturing
Corrosion resistance
It is provided by the presence natural oxide layer on the surface
because of the high reactivity with the atmosphere. In chemical terms, these metals are called passivable and in particular they are Al, Ti, Cr or Si.
For high-temperature applications, the corrosion resistance is harder
to keep, and sometimes a protective coating may be applied
Toughness and ductility
Limited ductility - toughness at low temperatures
The two major brittle failure modes - cleavage and inter-granular
fracture Plastic deformation is more difficult in intermetallic than in conventional alloys because of the stronger atomic bounding and the consequent ordered atomic distribution
11/7/2012
J.Muhammad Thanzeem Ibrahim M.Tech Manufacturing
Types
Beryllides
The Be-rich phases with Ti, Zr, Hf, Nb, Ta, or Mo, also called transition-metal
beryllides
BeO layer on the surface of the material comported a good oxidation resistance,
therefore Be-rich phases are also used as protective
Silicides
Silicides are considerate as an intermediate case between ceramics and metals. For example, transition metal silicides are often classed as intermetallic nevertheless silicon is a semiconductor and not a metal. Crystal structure of silicon is shown
Eg:M3Si, M2Si, M5Si3, MSi
Crystal structure of silicon and MoSi2
11/7/2012
J.Muhammad Thanzeem Ibrahim M.Tech Manufacturing
Rare-earth compounds
Rare-earth compounds have larger sizes and lower electronegativities The crystal structure derive from the cubic D21 structure or hexagonal
D2d structure Mostly of the rare-earth compounds are line compound, so that they have only narrow composition ranges. The production of these compounds is very difficult because of the high affinity with oxygen that leads to reaction with other commons materials. Magnetic properties are the most important property of rare-earth compound and these depend on the composition and crystal structure
11/7/2012
J.Muhammad Thanzeem Ibrahim M.Tech Manufacturing
Production:
Impossible to machine, due to brittleness at room temperature Hence they are produced by powder metallurgy Pressed pellets of a stoichiometric mixture were ignited. When heated to their transition temperatures, they will compact
themselves which is ordinarily well below their melting points.
Their reactivity allows alloying to occur at substantially reduced
temperatures. Eg: The Beryllides with Nb, Zr, or similar transition metals
11/7/2012
J.Muhammad Thanzeem Ibrahim M.Tech Manufacturing
10
Fracture mode
Intermetallic compounds have three different modes of fracture such
as: inter-granular fracture, trans-granular cleavage and a mixed of these first modes (see fig.1). The more thickness the sample the higher probability to have inter-granular fracture.
a)Inter-granular fracture(IF) b)Trans-granular fracture (TG) c) IF +TG.
11/7/2012
J.Muhammad Thanzeem Ibrahim M.Tech Manufacturing
11
Advantages
High hardness High wear resistance Low toughness
Low ductility
Good oxidation resistance High-temperature strength Microstructural stability
Disadvantage
Brittle at room temperature - a major obstacle to use in structural
applications Lack the appropriate balance of properties needed in engineering materials
11/7/2012
J.Muhammad Thanzeem Ibrahim M.Tech Manufacturing
12
APPLICATIONS OF INTERMETALLIC COMPOUNDS
Solder joints
Three commonly found intermetallic compounds in solder
joints are Cu6Sn5, Cu3Sn, and Ni3Sn4
Batteries
A large family of hydride-forming intermetallic compounds
that led to use in Ni-metal hydride batteries.
NiMH batteries comprise more than 30% of a $6 billion
market for rechargeable batteries used in many portable electronic devices such as cell phones and laptop computers.
11/7/2012
J.Muhammad Thanzeem Ibrahim M.Tech Manufacturing
13
Advantages of NiMH batteries
Higher storage capacity than Pb-acid and Ni-Cd batteries, Less toxicity than lead and cadmium Lower cost than Li-ion batteries
Magnets
Intermetallic compounds, including FeCo and rare earth
compounds, have been used as permanent magnets. Nd2Fe14B has the highest energy product of commercial permanent magnets.
11/7/2012
J.Muhammad Thanzeem Ibrahim M.Tech Manufacturing
14
REFERENCES
Sauthoff, G.,Intermetallics, VCH (1995). Fhnle, M., Welsch, F., From the electronic structure to the
macroscopic magnetic behaviour of rare-earth intermetallics, Physica B 321 (2002), 198-203. Kimura Y., Pope, D.P., Ductility and toughness in intermetallics, Intermetallics 6 (1998), 567-571. Fields R. J., Low S. R., Physical and mechanical properties of intermetallics compounds commonly found in solder joints, Metallurgy Division of MSEL (2002) Slotoff, N. S.; Liu, C. T.; Deevi, S. C. Emerging applications of intermetallics. Intermetallics (2000), 8(9-11), 1313-1320. Center for computational materials science, description of general behaviour of materials, cst-www.nrl.navy.mil. Crystalline structure of elements, www.geo.arizona.edu
Vous aimerez peut-être aussi
- Engineering Corrosion OH-7: University of Hafr Al BatinDocument27 pagesEngineering Corrosion OH-7: University of Hafr Al BatinHussain Al-DawoodPas encore d'évaluation
- Environment and CorrosionDocument39 pagesEnvironment and Corrosionabdullah anwarPas encore d'évaluation
- SMAWDocument40 pagesSMAWKoushik SarkarPas encore d'évaluation
- AbloDocument10 pagesAbloSmit DavePas encore d'évaluation
- Engineering Materials MEE 110: Dr. M. Tauqeer AnwarDocument13 pagesEngineering Materials MEE 110: Dr. M. Tauqeer AnwarAli HassanPas encore d'évaluation
- Properties of Intermetallics: Presented By: RAM KRISHNA (2018UGMM075) Satya Prakash Sahoo (2018ugmm076)Document15 pagesProperties of Intermetallics: Presented By: RAM KRISHNA (2018UGMM075) Satya Prakash Sahoo (2018ugmm076)Ram KrishnaPas encore d'évaluation
- Process Equipment DesignDocument3 pagesProcess Equipment DesignakshaylattimardiPas encore d'évaluation
- SeminarDocument12 pagesSeminarRaunak RajpalPas encore d'évaluation
- Corrosion - JUDocument32 pagesCorrosion - JUSWAGATAM BAZPas encore d'évaluation
- Lec 2 Material Science and EngineeringDocument13 pagesLec 2 Material Science and EngineeringMaina PhysicistPas encore d'évaluation
- Controlofcorrosionofunderwaterpiles 141108155314 Conversion Gate01Document34 pagesControlofcorrosionofunderwaterpiles 141108155314 Conversion Gate01Vinod KumarPas encore d'évaluation
- Engineering Materials: Mechanism in MetalsDocument38 pagesEngineering Materials: Mechanism in Metalssamurai7_77Pas encore d'évaluation
- CORROSIONDocument19 pagesCORROSIONTH Rongon100% (1)
- Advanced Structural CeramicsDocument12 pagesAdvanced Structural CeramicsKarthick RamPas encore d'évaluation
- Chapter 5 - CORROSION AND NON-FERROUS METALDocument60 pagesChapter 5 - CORROSION AND NON-FERROUS METALتاج نيسها33% (3)
- EME Module 2Document31 pagesEME Module 2Yashaswini AnandPas encore d'évaluation
- Engineering Corrosion OH-5: University of Hafr Al BatinDocument23 pagesEngineering Corrosion OH-5: University of Hafr Al BatinHussain Al-DawoodPas encore d'évaluation
- Engg Materials I FACDocument36 pagesEngg Materials I FACmhk665Pas encore d'évaluation
- CH 1 IntroductionDocument10 pagesCH 1 IntroductionSezin AYHANPas encore d'évaluation
- Metal Corrosion and Its Prevention: Material ScienceDocument49 pagesMetal Corrosion and Its Prevention: Material Sciencedr nfPas encore d'évaluation
- Biomaterials and Implants (Bmeg3183) 2.1 Metallic BiomaterialDocument50 pagesBiomaterials and Implants (Bmeg3183) 2.1 Metallic BiomaterialZeynu Kulizeyn100% (1)
- Venkatesan 1995Document14 pagesVenkatesan 1995YAZID BUSTOMIPas encore d'évaluation
- Forms of Corrosion: CHE-545-172 DR Ime B.ObotDocument17 pagesForms of Corrosion: CHE-545-172 DR Ime B.ObotAnonymous NxpnI6jCPas encore d'évaluation
- Lecture 1 - Introduction To Materials ScienceDocument32 pagesLecture 1 - Introduction To Materials ScienceGR footballPas encore d'évaluation
- HAZ Egycorr2015Document15 pagesHAZ Egycorr2015sajjadPas encore d'évaluation
- 1.2 CHAP 1 and CHAP2 Lecture 1Document28 pages1.2 CHAP 1 and CHAP2 Lecture 1Business MatterPas encore d'évaluation
- Effect of Anodization On The Corrosion Behavior of Aluminium Alloy in HCL Acid and NaohDocument5 pagesEffect of Anodization On The Corrosion Behavior of Aluminium Alloy in HCL Acid and NaohGabriel DevaPas encore d'évaluation
- Corrosion MechanismDocument33 pagesCorrosion MechanismMD IMRAN HOSSENPas encore d'évaluation
- CorrosionDocument19 pagesCorrosionIsrael HailuPas encore d'évaluation
- Galvanic, Concentration and Pitting CorrosionDocument42 pagesGalvanic, Concentration and Pitting CorrosionLeeMayYanPas encore d'évaluation
- EMS 223 Chapter 1Document38 pagesEMS 223 Chapter 1charles makasabiPas encore d'évaluation
- Production Methods of Laminated Al Alloy Composites: Current Trends in Civil & Structural EngineeringDocument3 pagesProduction Methods of Laminated Al Alloy Composites: Current Trends in Civil & Structural EngineeringأبومحمدالزياتPas encore d'évaluation
- Electroplating IIDocument53 pagesElectroplating IIGarima UppadhyayPas encore d'évaluation
- 2023MSEII Handout9Document12 pages2023MSEII Handout9王竣右Pas encore d'évaluation
- Course Content: No. Title Slide NoDocument54 pagesCourse Content: No. Title Slide NoDilip YadavPas encore d'évaluation
- Materials 1stDocument24 pagesMaterials 1stSaiful IslamPas encore d'évaluation
- Effects of Different Parameters On Molybdenum Concentration in ZN MO MN AlloyDocument7 pagesEffects of Different Parameters On Molybdenum Concentration in ZN MO MN AlloyEditor IJTSRDPas encore d'évaluation
- Corrosion 2 PDFDocument46 pagesCorrosion 2 PDFEenadu paperPas encore d'évaluation
- Evironmental Degradation of Materials: Rushikesh KulkarniDocument30 pagesEvironmental Degradation of Materials: Rushikesh KulkarniSanketDesai100% (1)
- Metals Used in ProsthodonticsDocument43 pagesMetals Used in ProsthodonticsnavneetkhanPas encore d'évaluation
- Kelompok 4 - Classifications of Materials - PPTDocument15 pagesKelompok 4 - Classifications of Materials - PPTAnnas HanifPas encore d'évaluation
- Electro FormingDocument16 pagesElectro Formingfrans william100% (1)
- CorroisionDocument21 pagesCorroisionIrtiza RasulPas encore d'évaluation
- Knowledge Challenger-June'16: Diffusion Engineers LimitedDocument4 pagesKnowledge Challenger-June'16: Diffusion Engineers LimitedSandeep NehraPas encore d'évaluation
- Ceramics:: Covalent Bonds: Are Formed by Electrons Shared Between Adjacent Atoms. These AreDocument14 pagesCeramics:: Covalent Bonds: Are Formed by Electrons Shared Between Adjacent Atoms. These ArebobpasxalPas encore d'évaluation
- Unit 2 - Manufacturing Process - WWW - Rgpvnotes.inDocument11 pagesUnit 2 - Manufacturing Process - WWW - Rgpvnotes.inIPL 2020 LIVE100% (1)
- Corrosion PresentationDocument51 pagesCorrosion PresentationJawad AslamPas encore d'évaluation
- Mewt - Eta - Submitted by AnanyasriDocument32 pagesMewt - Eta - Submitted by Ananyasriannem ananyasriPas encore d'évaluation
- Corrosion & Non-Ferrous MetalDocument21 pagesCorrosion & Non-Ferrous Metalsiraphat.bmPas encore d'évaluation
- Metallurgy Section: Jaramilla, John Erl SDocument25 pagesMetallurgy Section: Jaramilla, John Erl SMaynard Trinidad MendozaPas encore d'évaluation
- Anodic Protection: Liquid Environment: Name: Muhammad Emir Rafiansyah Akbar NPM: 2006489193Document30 pagesAnodic Protection: Liquid Environment: Name: Muhammad Emir Rafiansyah Akbar NPM: 2006489193emir akbarPas encore d'évaluation
- Cases Study For Corrosion in Heat Affected Zone of Carbon SteelDocument15 pagesCases Study For Corrosion in Heat Affected Zone of Carbon Steelmohamed al-amirPas encore d'évaluation
- Pitting CorrosionDocument188 pagesPitting CorrosionJosé Ramírez100% (5)
- Introduction To Material Science and MetallurgyDocument28 pagesIntroduction To Material Science and Metallurgytani nPas encore d'évaluation
- PolymersDocument30 pagesPolymersIrfan DanialPas encore d'évaluation
- WINSEM2018-19 - CHY1701 - ETH - SJT304 - VL2018195004125 - Reference Material I - EC - Module-4-Corrosion ProtectionDocument61 pagesWINSEM2018-19 - CHY1701 - ETH - SJT304 - VL2018195004125 - Reference Material I - EC - Module-4-Corrosion ProtectionkumarklPas encore d'évaluation
- W-2&3 Corrosion and Its TypesDocument46 pagesW-2&3 Corrosion and Its TypesUsamaPas encore d'évaluation
- 1st AssessDocument45 pages1st Assessمحمد تانزيم ابراهيمPas encore d'évaluation
- When The Recommended Fast That Coincides With A SaturdayDocument3 pagesWhen The Recommended Fast That Coincides With A Saturdayمحمد تانزيم ابراهيمPas encore d'évaluation
- chp10 PDFDocument23 pageschp10 PDFمحمد تانزيم ابراهيمPas encore d'évaluation
- Experimental ObjectiveDocument17 pagesExperimental Objectivependrive80Pas encore d'évaluation
- Front PageDocument3 pagesFront Pageمحمد تانزيم ابراهيمPas encore d'évaluation
- What Is Al Hakimyah PDFDocument2 pagesWhat Is Al Hakimyah PDFمحمد تانزيم ابراهيمPas encore d'évaluation
- Anna University CGPA To PercentageDocument1 pageAnna University CGPA To Percentageمحمد تانزيم ابراهيمPas encore d'évaluation
- ME2155 - Computer Aided Drafting & Modeling LaboratoryDocument73 pagesME2155 - Computer Aided Drafting & Modeling Laboratoryمحمد تانزيم ابراهيمPas encore d'évaluation
- Rankine Cycle SumDocument76 pagesRankine Cycle Sumمحمد تانزيم ابراهيم100% (1)
- Engineering Thermodynamics (May2013)Document3 pagesEngineering Thermodynamics (May2013)Siva2sankarPas encore d'évaluation
- Chpt6 Wal-Mart and Mattel PresentationDocument38 pagesChpt6 Wal-Mart and Mattel Presentationمحمد تانزيم ابراهيمPas encore d'évaluation
- Anna University College CodesDocument9 pagesAnna University College CodesIsaac John RaviPas encore d'évaluation
- Supply Chain Management (3rd Edition)Document31 pagesSupply Chain Management (3rd Edition)Najoua BoukriPas encore d'évaluation
- Thermodynamics 2 E7Document41 pagesThermodynamics 2 E7taya699Pas encore d'évaluation
- Pad Eye, Solidwork - TutorialDocument28 pagesPad Eye, Solidwork - TutorialJogi Oscar SinagaPas encore d'évaluation
- Dead Supplicting For LivingDocument2 pagesDead Supplicting For Livingمحمد تانزيم ابراهيمPas encore d'évaluation
- Thermodynamics 2 E7Document41 pagesThermodynamics 2 E7taya699Pas encore d'évaluation
- Wal MartDocument0 pageWal MartNirmal PatelPas encore d'évaluation
- Supply Chain ManagementDocument31 pagesSupply Chain ManagementAnonymous wA6NGuyklDPas encore d'évaluation
- Me 1201 - Engineering Thermodynamics (3rd Sem. Mech.)Document22 pagesMe 1201 - Engineering Thermodynamics (3rd Sem. Mech.)محمد تانزيم ابراهيمPas encore d'évaluation
- Usepa - 3051 ADocument30 pagesUsepa - 3051 Awrangel_2Pas encore d'évaluation
- Abdul Ilaah LahmaameeDocument1 pageAbdul Ilaah Lahmaameeمحمد تانزيم ابراهيمPas encore d'évaluation
- What Should The Husband Do On The First Night With His WifeDocument1 pageWhat Should The Husband Do On The First Night With His Wifeمحمد تانزيم ابراهيمPas encore d'évaluation
- Saudi Fatwa On Usaamah Ibn Laadin Al KhaarijeeDocument17 pagesSaudi Fatwa On Usaamah Ibn Laadin Al KhaarijeeAamir MughalPas encore d'évaluation
- 12.identify Observing Implementing and Generating A New Product.Document1 page12.identify Observing Implementing and Generating A New Product.محمد تانزيم ابراهيمPas encore d'évaluation
- 6010C Metals in Solution by ICP-AES (EPA 2000)Document34 pages6010C Metals in Solution by ICP-AES (EPA 2000)محمد تانزيم ابراهيمPas encore d'évaluation
- 9.write The Short Notes On Laser ScanningDocument4 pages9.write The Short Notes On Laser Scanningمحمد تانزيم ابراهيمPas encore d'évaluation
- Muhammad Ash-Shanqeeti - Wikipedia, The Free Encyclopedia PDFDocument3 pagesMuhammad Ash-Shanqeeti - Wikipedia, The Free Encyclopedia PDFمحمد تانزيم ابراهيمPas encore d'évaluation
- Mechanical Properties of Date Palm Fiber Reinforced CompositesDocument9 pagesMechanical Properties of Date Palm Fiber Reinforced Compositesمحمد تانزيم ابراهيمPas encore d'évaluation
- EPA 3050b 1Document12 pagesEPA 3050b 1Giuseppe GoriPas encore d'évaluation
- IPC 2022-87302 Threat Assessment Considerations For Vintage Pipes - 87302 - FinalDocument10 pagesIPC 2022-87302 Threat Assessment Considerations For Vintage Pipes - 87302 - FinalOswaldo MontenegroPas encore d'évaluation
- Lecture 6-Behavior of Materials in ServiceDocument35 pagesLecture 6-Behavior of Materials in ServiceAtika AlamPas encore d'évaluation
- Analysis of Longitudinal Timber Beam Joints Loaded With Simple BendingDocument15 pagesAnalysis of Longitudinal Timber Beam Joints Loaded With Simple BendingsxturboPas encore d'évaluation
- Folds, Faults, and The Deformation of Earth's CrustDocument34 pagesFolds, Faults, and The Deformation of Earth's CrustReliza Amahan SenoPas encore d'évaluation
- Chapter 4: Imperfections in The Atomic and Ionic ArrangementsDocument20 pagesChapter 4: Imperfections in The Atomic and Ionic ArrangementsMarcos JosePas encore d'évaluation
- Construction and Building Materials: Mohammed Farooq, Nemkumar BanthiaDocument12 pagesConstruction and Building Materials: Mohammed Farooq, Nemkumar BanthiaTuğçe VuralPas encore d'évaluation
- Unit-II EME AerospaceDocument51 pagesUnit-II EME AerospacePranil Vijay SawalakhePas encore d'évaluation
- Chapter Outline: Failure: Principles of Fracture MechanicsDocument34 pagesChapter Outline: Failure: Principles of Fracture MechanicsAldrin BendalPas encore d'évaluation
- Pourmohammad 2019Document11 pagesPourmohammad 2019CHONKARN CHIABLAMPas encore d'évaluation
- ASME Sec VIII - AwarenessDocument33 pagesASME Sec VIII - AwarenessNirmal100% (4)
- Vulnerable To Brittle FractureDocument7 pagesVulnerable To Brittle FractureZenon KociubaPas encore d'évaluation
- Exit ExamDocument70 pagesExit ExamHaymanot MelakuPas encore d'évaluation
- Brittleness and Rock Strength of The Bakken Formation, Williston Basin, North DakotaDocument20 pagesBrittleness and Rock Strength of The Bakken Formation, Williston Basin, North DakotaJimmy100% (1)
- Fracture - Material TechnologyDocument18 pagesFracture - Material TechnologyayushdbcPas encore d'évaluation
- Lecture VVI 24 3 2020 10 40-12 40 Chitra Physics Electronics UPI-32511204Document10 pagesLecture VVI 24 3 2020 10 40-12 40 Chitra Physics Electronics UPI-32511204Chaitali KutePas encore d'évaluation
- Fracture Mechanics Lecture SlidesDocument37 pagesFracture Mechanics Lecture SlidesCrystal WoodsPas encore d'évaluation
- CE 203 Civil Engineering Materials: Dr. Abdulrahman K. YussefDocument11 pagesCE 203 Civil Engineering Materials: Dr. Abdulrahman K. Yussefعبدالرحمن الحازميPas encore d'évaluation
- Brittle Fracture The Cold Hard Facts Vern Ragle FinalDocument42 pagesBrittle Fracture The Cold Hard Facts Vern Ragle FinalCharle MathewPas encore d'évaluation
- Avoid Brittle Fracture in Pressure VesselsDocument7 pagesAvoid Brittle Fracture in Pressure VesselsSajal KulshresthaPas encore d'évaluation
- 2 Marks QuestionsDocument30 pages2 Marks QuestionsGanesh RPas encore d'évaluation
- Chap 03Document42 pagesChap 03Americo MolinaPas encore d'évaluation
- Earth Science 2nd Quarter Module 2Document8 pagesEarth Science 2nd Quarter Module 2Creepy GrinPas encore d'évaluation
- 11212021091341M3 EARTH SCIENCE Deformation of The Earths CrustDocument14 pages11212021091341M3 EARTH SCIENCE Deformation of The Earths CrustJhon PerezPas encore d'évaluation
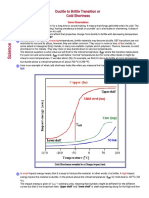
- Ductile To Brittle Transition or Cold ShortnessDocument12 pagesDuctile To Brittle Transition or Cold Shortnessvamsi patnalaPas encore d'évaluation
- List of Mechanical Properties of MaterialsDocument5 pagesList of Mechanical Properties of MaterialsS GPas encore d'évaluation
- Types of Welding in ShipbuildingDocument30 pagesTypes of Welding in ShipbuildingPranjyoti SaikiaPas encore d'évaluation
- 05 Introduction of Fracture MechanicsDocument24 pages05 Introduction of Fracture MechanicsMallika ChowdaryPas encore d'évaluation
- Q1 Summative and Performance TaskDocument17 pagesQ1 Summative and Performance Taskimelda d. lampaPas encore d'évaluation
- Define: Brittle FractureDocument9 pagesDefine: Brittle Fractureskaaareeee2004Pas encore d'évaluation
- CM-II Impact Strength ReportDocument5 pagesCM-II Impact Strength ReportLowKey HumanPas encore d'évaluation