Académique Documents
Professionnel Documents
Culture Documents
Tugas
Transféré par
Melanie WibowoTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Tugas
Transféré par
Melanie WibowoDroits d'auteur :
Formats disponibles
Example
A 0,467 g sample containing sodium bicarbonate (a monoacidic base) and titrated with a standard solution of HCl, requairing 40,72 ml. The hydrocloric acid was standarized by titrating 0,1876 g sodium carbonate, wich required 37,86 ml. Calculate the percent sodium bicarbonate in the sample. Solution : NHCl = meq NaHCO3 = mg/BE (BM) mL HCl mL
Persen NaHCO3 = ....................
Problems
Calculate the molar concentration of all the cations and anions in a solution prepared by mixing 10.0 mL each of the following solutions: 0.100 M Mn(NO3)2, 0.100 M KNO3, 0.100 M K2SO4. Calculate the grams of each substance required to prepare the following solution: (a) 250 mL of 0.100 M KOH (b) 1.00L of 0.0275 M K2Cr2O7 (c) 500 mL of 0.500 M CuSO4 How many milliliters of concentrated hydrochloric acid, 38% (wt/wt), specific gravity 1.19, are required to prepare 1L of a 0.100 M solution
Solution
Problems
How many milliliters of 0.10 M of H2SO4 must be added to 50 mL of 0.1 M NaOH to give a solution that is 0.050 M in H2SO4? Assumes volumes are additive.
Solution
Problems
A preparation of soda ash is known to contain 98.6% Na2CO3. If a 0.678 g sample requires 36.8 ml of sulfuric acid to complete neutralization, what is the molarity of the sulfuric acid solution? A sample of USP grade citric acid (H3C6O7, three titratable protons) is analyzed by titrating with 0.1087 M NaOH. If a 0.2678 g sample requires 38.31 ml for titration, what is the purity of the preparation? (USP requires 99.5%)
Solution
Problems
A solution is prepared by dissolving 7.82 g NaOH and 9.26 g Ba(OH)2 in water and diluting to 500 ml. What is the normality of the solution as a base? What weight of arsenic trioxide, As2O3, is required to prepare 1L of 0.1000 N arsenic (III) solution? The sulfur content of steel sample is determined by converting it to H2S gas, absorbing the H2S in 10 ml of 0.00500 M I2, and then back titrating the excess I2 with 0.00200M Na2S2O3. If 2.6 ml Na2S2O3 is required for titration, how many milligrams of sulfur are contained in the sample? A potassium permanganate solution is prepared by dissolving 4.68 g KMnO4 in water and diluting to 500 ml. How many milliliters of this will react with the iron in 0.500 g of ore containing 35.6% Fe2O3?
Solution
Vous aimerez peut-être aussi
- Quiz - Coordination Compounds PDFDocument2 pagesQuiz - Coordination Compounds PDFAman JaiswalPas encore d'évaluation
- 02 BaOH2 and H2SO4 Conductimetric Titration STUDENTDocument4 pages02 BaOH2 and H2SO4 Conductimetric Titration STUDENTargoniodidePas encore d'évaluation
- Experiment I1 Preparation of Some Cobaltammine ComplexesDocument7 pagesExperiment I1 Preparation of Some Cobaltammine ComplexesIftitah HauriyahPas encore d'évaluation
- Application of Neutralization TitrationsDocument21 pagesApplication of Neutralization TitrationsAsuncion Thea50% (2)
- 201652612114Document18 pages201652612114VismayPas encore d'évaluation
- VOLUMETRIC AnalysisDocument49 pagesVOLUMETRIC AnalysisLisa Dea SaryPas encore d'évaluation
- 2 (G) 2 (G) 2 (L) F 2 (L) - 1 (S) 2 (G) 2 (G) F 2 (G) - 1Document27 pages2 (G) 2 (G) 2 (L) F 2 (L) - 1 (S) 2 (G) 2 (G) F 2 (G) - 1SMJK KatholikPas encore d'évaluation
- Physical Chemistry OBJECTIVEDocument188 pagesPhysical Chemistry OBJECTIVEGadde Gopala Krishna100% (2)
- Expts For Chem EnggDocument37 pagesExpts For Chem Enggblackbeauty140% (1)
- Conversion of Concentration UnitsDocument9 pagesConversion of Concentration UnitsMustafa KhandgawiPas encore d'évaluation
- Acid Base Titration (Theory) - Inorganic Chemistry Virtual Lab - Chemical Sciences - Amrita Vishwa Vidyapeetham Virtual LabDocument8 pagesAcid Base Titration (Theory) - Inorganic Chemistry Virtual Lab - Chemical Sciences - Amrita Vishwa Vidyapeetham Virtual Labpankaj111Pas encore d'évaluation
- Acid Base Titration ExperimentDocument2 pagesAcid Base Titration ExperimentDark_KiroPas encore d'évaluation
- Atomic Structure and Spectra: Selection RulesDocument51 pagesAtomic Structure and Spectra: Selection RulesAdministracion OTIC IVICPas encore d'évaluation
- Applications of Redox ReactionsDocument50 pagesApplications of Redox ReactionsMlamuli MlarhPas encore d'évaluation
- Redox TitrationDocument31 pagesRedox Titrationحمامة السلامPas encore d'évaluation
- Chapter 06 Phase Equilibria 4 PDF FreeDocument77 pagesChapter 06 Phase Equilibria 4 PDF FreeGabriel SilvaPas encore d'évaluation
- GC-MS Quiz - RobDocument10 pagesGC-MS Quiz - Robchegu BusinessPas encore d'évaluation
- Exp 2 - DilutionDocument6 pagesExp 2 - DilutionSiti FatimahPas encore d'évaluation
- Standardization of Naoh 1Document3 pagesStandardization of Naoh 1api-309208977Pas encore d'évaluation
- 8 - Sample Titration ProblemsDocument15 pages8 - Sample Titration ProblemsGerald LimPas encore d'évaluation
- Inorganic Qualitative Analysis Acidic RadicalDocument24 pagesInorganic Qualitative Analysis Acidic RadicalShivani ShreshthaPas encore d'évaluation
- Chemical Kinetics Reaction Mechanism Frederick LindemannDocument10 pagesChemical Kinetics Reaction Mechanism Frederick LindemannSaman AkramPas encore d'évaluation
- Punjab College Pattoki: Spring 2021: Course Outline Bs Program Semester 6ThDocument8 pagesPunjab College Pattoki: Spring 2021: Course Outline Bs Program Semester 6ThFareeha ShakeelPas encore d'évaluation
- Titration Sample ProblemDocument8 pagesTitration Sample ProblemPaulAcademicsPas encore d'évaluation
- Metal ClustersDocument23 pagesMetal ClustersSyed Safi AhmedPas encore d'évaluation
- Sample Question 3 With AnswerDocument18 pagesSample Question 3 With AnswerPyae Sone Kyaw100% (1)
- Redox Titration QuizDocument1 pageRedox Titration QuizChen Lit YangPas encore d'évaluation
- Methods of Presenting Concentration - Lecture 2Document31 pagesMethods of Presenting Concentration - Lecture 2Acidri Abdulkarim100% (1)
- HPLC NotesDocument50 pagesHPLC NotesEmmanuella Offiong100% (1)
- Chapter 5a.doc S.U.G and Girls PDFDocument53 pagesChapter 5a.doc S.U.G and Girls PDFKanagaraj ArumugamPas encore d'évaluation
- Acid Neutralizing Capacity of An Antacid: BackgroundDocument5 pagesAcid Neutralizing Capacity of An Antacid: BackgroundNabilah HarisPas encore d'évaluation
- Preparation of SolutionsDocument39 pagesPreparation of SolutionsIrfan JanjuaPas encore d'évaluation
- Inorganic Chemistry D-Block ElementsDocument19 pagesInorganic Chemistry D-Block ElementsshinyeePas encore d'évaluation
- Chapter 4rth Liquids and Solids McqsDocument6 pagesChapter 4rth Liquids and Solids McqsHaider JalalPas encore d'évaluation
- Lab SolutionsDocument28 pagesLab SolutionsRaja Mohan Gopalakrishnan0% (1)
- Module 1 SPINELDocument5 pagesModule 1 SPINELDharmendra Kumar SrivastavaPas encore d'évaluation
- Acid Dissociation ConstantDocument4 pagesAcid Dissociation ConstantJair RangelPas encore d'évaluation
- Microwave Infrared: SpectrosDocument66 pagesMicrowave Infrared: SpectrosPrathamesh Dash100% (2)
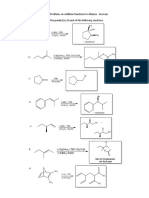
- Practice Problems On Addition Reactions To Alkenes With AnswersDocument4 pagesPractice Problems On Addition Reactions To Alkenes With AnswersSangetha ChelladoraiPas encore d'évaluation
- Absorption Laws (Quantitative Analysis)Document15 pagesAbsorption Laws (Quantitative Analysis)Belay HailePas encore d'évaluation
- Back TitrateDocument16 pagesBack Titratepicket1019Pas encore d'évaluation
- Empirical and Molecular Formulae WorksheetDocument3 pagesEmpirical and Molecular Formulae WorksheetJohnclyde Ferry100% (1)
- 6 Good Tritration PDFDocument5 pages6 Good Tritration PDFUjak KimiaPas encore d'évaluation
- Class 11 Chemistry Notes 2023-24 8. Redox ReactionsDocument40 pagesClass 11 Chemistry Notes 2023-24 8. Redox ReactionsAyushi Shah100% (1)
- Gain Familiarity With Some of The Acid-Base, Oxidation-Reduction and Complexion Reaction of The Elements of The First Transition Series.Document11 pagesGain Familiarity With Some of The Acid-Base, Oxidation-Reduction and Complexion Reaction of The Elements of The First Transition Series.FarahSyazwani100% (1)
- Titrimetric MethodsDocument34 pagesTitrimetric MethodsMuhdLuqmanPas encore d'évaluation
- Chapter 15 Parallels Between Main Group and Organometallic ChemistryDocument46 pagesChapter 15 Parallels Between Main Group and Organometallic ChemistryTarang BhatiPas encore d'évaluation
- Atmospheric Radicals (Lecture 2)Document7 pagesAtmospheric Radicals (Lecture 2)Qamer NisaPas encore d'évaluation
- Lab ManualDocument19 pagesLab Manualanon_467104036Pas encore d'évaluation
- C3 Titration CalculationsDocument1 pageC3 Titration CalculationsYuones BalahPas encore d'évaluation
- Qualitative Organic AnalysisDocument24 pagesQualitative Organic AnalysisSofia FuentesPas encore d'évaluation
- EP103 Sen LNT 003d Sep11Document16 pagesEP103 Sen LNT 003d Sep11Sàtz ÑÖÑït0% (1)
- Volumetric AnalysisDocument4 pagesVolumetric AnalysiskuthappadyPas encore d'évaluation
- Iodimetric Titration: Aim: PrincipleDocument2 pagesIodimetric Titration: Aim: PrincipleHarsh ThakurPas encore d'évaluation
- Types of ElectrolytesDocument95 pagesTypes of ElectrolytesDeepak Sirone100% (4)
- Lecture 6 Kinetic Isotope EffectDocument11 pagesLecture 6 Kinetic Isotope EffectcsnPas encore d'évaluation
- Enzymes: A Practical Introduction to Structure, Mechanism, and Data AnalysisD'EverandEnzymes: A Practical Introduction to Structure, Mechanism, and Data AnalysisÉvaluation : 4 sur 5 étoiles4/5 (2)
- ChemistryDocument2 pagesChemistryniloPas encore d'évaluation