Académique Documents
Professionnel Documents
Culture Documents
A B G Asu
Transféré par
FathiBestTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
A B G Asu
Transféré par
FathiBestDroits d'auteur :
Formats disponibles
Prof. Dr.
Heba Halawa
Overview
The pH is a measurement of the acidity or
alkalinity of the blood.
It is inversely proportional to the number of
hydrogen ions (H+) in the blood
The pH of a solution is measured on a scale
from 1 (very acidic) to 14 (very alkalotic).
A liquid with a pH of 7, such as water, is
neutral (neither acidic nor alkalotic).
The normal blood pH range is 7.35 to 7.45
When the pH is below 7.35, the blood is said to
be acidic.
Effects of Acidic pH
Decrease in the force of cardiac contractions
Decrease in the vascular response to catecholamines
When the pH is above 7.45, the blood is said to
be alkalotic.
An alkalotic state interferes with tissue
oxygenation and normal neurological and
muscular functioning.
Significant changes in the blood pH above 7.8 or
below 6.8 will interfere with cellular
functioning, and if uncorrected, will lead to
death.
A normal by-product of cellular metabolism is
carbon dioxide (CO2).
CO2 is carried in the blood to the lungs, where
excess CO2 combines with water (H2O) to form
carbonic acid (H2CO3). The blood pH will change
according to the level of carbonic acid present.
This triggers the lungs to either increase or
decrease the rate and depth of ventilation until
the appropriate amount of CO2 has been re-
established.
Activation of the lungs to compensate for an
imbalance starts to occur within 1 to 3 minutes.
In an effort to maintain the pH of the blood
within its normal range, the kidneys excrete
or retain bicarbonate (HCO3-).
As the blood pH decreases, the kidneys will
compensate by retaining HCO3- and as the
pH rises, the kidneys excrete HCO3- through
the urine.
Although the kidneys provide an excellent
means of regulating acid-base balance, the
system may take from hours to days to
correct the imbalance.
Normal ABGs Values
Ph: 7.35-7.45
PaCO2: 35-45 mmHg
PaO2: 80-100 mmHg
HCO3: 22-26 meq/L
SaO2: > 95%
Step One
Assess the pH
Above 7.45, the blood is alkalotic.
Below 7.35, the blood is acidotic.
Step Two
If the blood is alkalotic or acidotic, we now
need to determine if it is caused primarily by
a respiratory or metabolic problem.
To do this, assess the PaCO2 level. Remember
that with a respiratory problem, as the pH
decreases below 7.35, the PaCO2 should rise.
If the pH rises above 7.45, the PaCO2 should
fall.
Step Three
Finally, assess the HCO3 value. Recall that
with a metabolic problem, normally as the
pH increases, the HCO3 should also increase.
Likewise, as the pH decreases, so should the
HCO3.
pH PaCO2 HCO3
Respiratory
Acidosis
variable
Respiratory
Alkalosis
variable
Metabolic
Acidosis
variable
Metabolic
Alkalosis
variable
Causes
Renal Failure
Diabetic ketoacidosis
Alcoholic ketoacidosis
Lactic acidosis
Poisonings
Salicylates
Ethylene glycol
Methanol
Iron
pH: < 7.35
HCO3: < 22 meq/L
PaCO2: variable
Causes
I- Respiratory depression
Opiates
Sedatives
II- Respiratory muscle
weakness
OPC
Botulism
Paralytic snakes
III- Pulmonary edema
pH: < 7.35
PaCO2: > 45 mmHg
HCO3: Variable
Causes
Gastric acid loss: vomiting
Excessive bicarbonate intake
pH: > 7.45
HCO3: > 28 meq/L
Causes
Acute hyperventilation e.g.
Early salicylate tox.
Early theophylline toxicity
Anxiety
pH: > 7.45
PaCO2: < 35 mmHg
HCO3: Variable
pH 7.33 PaCO2 60 HCO3 34
A. Normal ABG values
B. Respiratory acidosis without compensation
C. Respiratory acidosis with partial
compensation
D. Respiratory acidosis with full compensation
pH 7.48 PaCO2 42 HCO3 30
A. Metabolic acidosis without compensation
B. Respiratory alkalosis with partial
compensation
C. Respiratory alkalosis with full compensation
D. Metabolic alkalosis without compensation
pH 7.38 PaCO2 38 HCO3 24
A. Respiratory alkalosis
B. Normal
C. Metabolic Alkalosis
D. None of the above
pH 7.21 PaCO2 60 HCO3 24
A. Normal
B. Respiratory acidosis without compensation
C. Metabolic acidosis with partial
compensation
D. Respiratory acidosis with complete
compensation
pH 7.45 PaCO2 26 HCO3 16
A. Normal
B. Respiratory acidosis fully compensated
C. Respiratory alkalosis fully compensated
D. Metabolic alkalosis fully compensated
pH 7.50 PaCO2 29 HCO3 24
A. Normal
B. Respiratory acidosis with compensation
C. Respiratory alkalosis without compensation
D. Metabolic alkalosis with partial
compensation
Vous aimerez peut-être aussi
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (588)
- Pensma Souvenir Programme & Abstract Book - 13Document15 pagesPensma Souvenir Programme & Abstract Book - 13FathiBestPas encore d'évaluation
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- History Taking - P.A.DDocument17 pagesHistory Taking - P.A.DFathiBestPas encore d'évaluation
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5795)
- EVC2019 Final Program PDFDocument29 pagesEVC2019 Final Program PDFFathiBestPas encore d'évaluation
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- 31oct2014 Investment StructureDocument1 page31oct2014 Investment StructureFathiBestPas encore d'évaluation
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (895)
- A LifeLinkDocument18 pagesA LifeLinkFathiBestPas encore d'évaluation
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (345)
- Clinical Sheets 2Document38 pagesClinical Sheets 2FathiBestPas encore d'évaluation
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- VISA Entry Requirements To MalaysiaDocument11 pagesVISA Entry Requirements To MalaysiaFathiBestPas encore d'évaluation
- Barista Guidelines - FIPDocument15 pagesBarista Guidelines - FIPFathiBest100% (1)
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (400)
- 2018 Bfa NatWest Franchise SurveyDocument28 pages2018 Bfa NatWest Franchise SurveyPapa Johny100% (1)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- Sf1 Cinderella JuneDocument60 pagesSf1 Cinderella JuneLaLa FullerPas encore d'évaluation
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- Inspection and Repair: Inspection of Totally Enclosed Lift Bags 1.0Document8 pagesInspection and Repair: Inspection of Totally Enclosed Lift Bags 1.0Ali KuliPas encore d'évaluation
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (74)
- Upstream Marine Standard IBU ChevronDocument52 pagesUpstream Marine Standard IBU ChevronAndrew Hendy Indrakusuma50% (2)
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- Understanding Building Construction Types - Firefighter Nation PDFDocument3 pagesUnderstanding Building Construction Types - Firefighter Nation PDFRakeshPas encore d'évaluation
- Central Banks Retail Forex DealersDocument7 pagesCentral Banks Retail Forex DealersBCom HonsPas encore d'évaluation
- Safety Protocols of SIMIS Inyerlocking SystemsDocument12 pagesSafety Protocols of SIMIS Inyerlocking SystemsNECDET ÇİLİNGİR100% (1)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (266)
- Soft Tissue Sarcoma Asia-PasificDocument13 pagesSoft Tissue Sarcoma Asia-PasificHayyu F RachmadhanPas encore d'évaluation
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- The Extinction of The DinosaursDocument42 pagesThe Extinction of The DinosaursIzzat BukhoriPas encore d'évaluation
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- Assignment - Certificate of Pre & Primary Teacher TrainingDocument4 pagesAssignment - Certificate of Pre & Primary Teacher TrainingSuganya VimalPas encore d'évaluation
- WEAKNESSESDocument3 pagesWEAKNESSESMarian grace DivinoPas encore d'évaluation
- Assessment A - Project Tasks (1) CompletedDocument5 pagesAssessment A - Project Tasks (1) Completednazifa zahid100% (2)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2259)
- Slaking SQ Physical Indicator SheetDocument2 pagesSlaking SQ Physical Indicator Sheetqwerty12348Pas encore d'évaluation
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1091)
- 02 TBS Lightning Protection System PDFDocument448 pages02 TBS Lightning Protection System PDFgilbertomjcPas encore d'évaluation
- O Feci : (BL (ElDocument107 pagesO Feci : (BL (ElKaren Elsy Gonzales CamachoPas encore d'évaluation
- MotherDocument11 pagesMotherMuhammad Lucky AlfaridzyPas encore d'évaluation
- Reference Texts - Textes de RéférenceDocument7 pagesReference Texts - Textes de RéférenceSid PatelPas encore d'évaluation
- Is 13428 2005Document47 pagesIs 13428 2005Varun DevPas encore d'évaluation
- W - Paul Smith, William C. Compton and W - Beryl WestDocument5 pagesW - Paul Smith, William C. Compton and W - Beryl Westefarfan26Pas encore d'évaluation
- Ayurvedic and Naturopathic Way of Life by Manthena Sathyanarayana RajuDocument7 pagesAyurvedic and Naturopathic Way of Life by Manthena Sathyanarayana RajuvishnuprakashPas encore d'évaluation
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (121)
- Intec Controls SPC31195 DatasheetDocument5 pagesIntec Controls SPC31195 DatasheetEduardo CalvaPas encore d'évaluation
- Obstetrical EmergencyDocument41 pagesObstetrical EmergencyDrPreeti Thakur ChouhanPas encore d'évaluation
- Rockland County Stream Biomonitoring ProjectDocument12 pagesRockland County Stream Biomonitoring ProjectHRNERRPas encore d'évaluation
- Roofing ActivitiesDocument2 pagesRoofing ActivitiesArnold Roy Coballes ManaloPas encore d'évaluation
- Power of The Mind 2010 PDFDocument32 pagesPower of The Mind 2010 PDFTijana Morača Aćimović100% (3)
- CUP 04 BOIN USA 02 WebDocument2 pagesCUP 04 BOIN USA 02 WebMajid abdulPas encore d'évaluation
- Latihan Soal Tentang Public PlacesDocument3 pagesLatihan Soal Tentang Public PlacesRiimha AmbarwatiPas encore d'évaluation
- July 16, 2016Document12 pagesJuly 16, 2016The Delphos HeraldPas encore d'évaluation
- A Strategic Behaviour Guidance Tool in Paediatric Dentistry: 'Reframing' - An ExperienceDocument3 pagesA Strategic Behaviour Guidance Tool in Paediatric Dentistry: 'Reframing' - An Experiencesilky groverPas encore d'évaluation
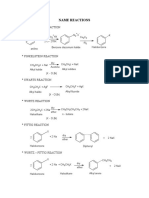
- Name Reactions: Sandmeyer'S ReactionDocument9 pagesName Reactions: Sandmeyer'S ReactionSai Krishnan100% (1)
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)