Académique Documents
Professionnel Documents
Culture Documents
Functions and Properties of Blood - Plasma - Blood Cell Production - Erythrocytes - Blood Types - Leukocytes - Hemostasis (Stoppage of Bleeding)
Transféré par
vanderphys0 évaluation0% ont trouvé ce document utile (0 vote)
104 vues47 pagesMCAT prep
Titre original
18 Blood
Copyright
© © All Rights Reserved
Formats disponibles
PPT, PDF, TXT ou lisez en ligne sur Scribd
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentMCAT prep
Droits d'auteur :
© All Rights Reserved
Formats disponibles
Téléchargez comme PPT, PDF, TXT ou lisez en ligne sur Scribd
0 évaluation0% ont trouvé ce document utile (0 vote)
104 vues47 pagesFunctions and Properties of Blood - Plasma - Blood Cell Production - Erythrocytes - Blood Types - Leukocytes - Hemostasis (Stoppage of Bleeding)
Transféré par
vanderphysMCAT prep
Droits d'auteur :
© All Rights Reserved
Formats disponibles
Téléchargez comme PPT, PDF, TXT ou lisez en ligne sur Scribd
Vous êtes sur la page 1sur 47
Chapter 18
The Circulatory System: Blood
Functions and properties of
blood
Plasma
Blood cell production
Erythrocytes
Blood types
Leukocytes
Hemostasis (stoppage of
bleeding)
Functions and Properties of Blood
Functions in respiration, nutrition, waste
elimination, thermoregulation, immune defense,
water and pH balance, etc.
Adults have 4-6 L of blood
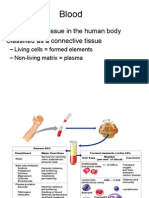
plasma, a clear extracellular fluid
formed elements (blood cells and platelets)
Properties of blood
viscosity (resistance to flow)
osmolarity (total molarity of dissolved particles)
if incorrect causes high BP or edema & low BP
Formed Elements of Blood
Hematocrit
Centrifuging blood forces formed elements to separate
from plasma
Hematocrit is % of total volume that is cells
Plasma and Plasma Proteins
Plasma is a mixture of proteins, enzymes, nutrients,
wastes, hormones, and gases
if allowed to clot, what remains is called serum
All plasma proteins formed by liver except globulins
(produced by plasma cells)
Albumins are most abundant plasma protein
contributes to viscosity and osmolarity and influences blood
pressure, flow and fluid balance
Fibrinogen is precursor of fibrin threads that help form
blood clots
Globulins provide immune system defenses
Nonprotein Components of Plasma
Plasma contains nitrogenous compounds
amino acids from dietary protein or tissue breakdown
nitrogenous wastes (urea) are toxic end products of
catabolism
normally removed from the blood by the kidneys
Nutrients (glucose, vitamins, fats, minerals, etc)
Some O
2
and CO
2
are transported in plasma
Many electrolytes are found in plasma
Na
+
makes up 90% of plasma cations accounting
for more of bloods osmolarity than any other
solute
Blood Cell Production (Hematopoiesis)
Hemopoietic tissues produce blood cells
yolk sac in vertebrate embryo produce stem cells that colonize
fetal bone marrow, liver, spleen & thymus
liver stops producing blood cells at birth, but spleen and thymus
remain involved with WBC production
lymphoid hemopoiesis occurs in widely distributed lymphoid
tissues (thymus, tonsils, lymph nodes, spleen & peyers patches in
intestines)
red bone marrow produces RBCs, WBCs and platelets
stem cells called hemocytoblasts multiply continually & are pluripotent
(capable of differentiating into multiple cell lines)
committed cells are destined to continue down one specific cell line
Stimulated by erythropoietin, thrombopoietin & colony-
stimulating factors (CSFs)
Hemopoiesis
Granulocytes
Erythrocyte Production
Erythropoiesis produces 2.5 million RBCs/second
First committed cell is proerythroblast
has receptors for erythropoietin (EPO) from kidneys
Erythroblasts multiply & synthesize hemoglobin
Nucleus degenerates to form a reticulocyte
named for fine network of endoplasmic reticulum
enters bloodstream as 0.5 to 1.5% of circulating RBCs
Development takes 3-5 days & involves
reduction in cell size, increase in cell number, synthesis of
hemoglobin & loss of nucleus
Erythrocyte Homeostasis
Classic negative feedback
control
drop in RBC count causes
hypoxemia to kidneys
erythropoietin production
stimulation of bone marrow
RBC count in 3-4 days
Stimulus for erythropoiesis
low levels of atmospheric O
2
increase in exercise
hemorrhaging
Nutritional Needs for Erythropoiesis
Iron is key nutritional requirement for erythropoiesis
lost daily through urine, feces, and bleeding
men 0.9 mg/day and women 1.7 mg/day
low absorption rate requires consumption of 5-20 mg/day
dietary iron in 2 forms: ferric (Fe
3+
) & ferrous (Fe
2+
)
stomach acid converts Fe+3 to absorbable Fe+2
gastroferritin from stomach binds Fe+2 & transports it to intestine
absorbed into blood & binds to transferrin to travel
bone marrow uses to make hemoglobin, muscle used to make
myoglobin and all cells use to make cytochromes in mitochondria
liver binds surplus to apoferritin to create ferritin for storage
B12 & folic acid (for rapid cell division) and vitamin C
& Cu for cofactors for enzymes synthesizing RBCs
Iron Absorption, Transport & Storage
Leukocyte Production (Leukopoiesis)
Committed cell types -- B & T progenitors and
granulocyte-macrophage colony-forming units
possess receptors for colony-stimulating factors
released by mature WBCs in response to infections
RBC stores & releases granulocytes & monocytes
Some lymphocytes leave bone marrow unfinished
go to thymus to complete their development (T cells)
Circulating WBCs do not stay in bloodstream
granulocytes leave in 8 hours & live 5 days longer
monocytes leave in 20 hours, transform into macrophages and
live for several years
WBCs providing long-term immunity last decades
Platelet Production (Thrombopoiesis)
Hemocytoblast that develops receptors for
thrombopoietin from liver or kidney becomes
megakaryoblast
Megakaryoblast repeatedly replicates its DNA
without dividing
forms gigantic cell called megakaryocyte (100 m in
diameter that remains in bone marrow)
Infoldings of megakaryocyte cytoplasm splits off
cell fragments that enter the bloodstream as
platelets (live for 4 days)
some stored in spleen & released as needed
Megakaryocytes & Platelets
Erythrocytes (RBCs)
Disc-shaped cell with thick rim
7.5 M diameter & 2.0 m thick at rim
Major function is gas transport
lost all organelles during maturation so
has increased surface area/volume ratio
increases diffusion rate of substances in & out of cell
33% of cytoplasm is hemoglobin (Hb)
O
2
delivery to tissue and CO
2
transport back to lungs
contains enzyme, carbonic anhydrase (CAH)
produces carbonic acid from CO2 and water
important role in gas transport & pH balance
Erythrocytes on a Needle
Hemoglobin Structure
Hemoglobin consists of 4
protein chains called
globins (2 alpha & 2 beta)
Each protein chain is
conjugated with a heme
group which binds oxygen
to ferrous ion (Fe+2)
Hemoglobin molecule can
carry four O2
Fetal hemoglobin
gamma instead of beta chains
Erythrocytes and Hemoglobin
RBC count & hemoglobin concentration indicate
the amount of oxygen the blood can carry
hematocrit is % of blood volume consisting of cells
men 42-52% cells; women 37-48% cells
hemoglobin concentration of whole blood
men 13-18g/dL; women 12-16g/dL
RBC count
men 4.6-6.2 million/L; women 4-2-5.4 million/L
Values are lower in women
androgens stimulate RBC production
women have periodic menstrual losses
Erythrocyte Death & Disposal
RBCs live for 120 days
membrane fragility -- lysis in
narrow channels in the spleen
Macrophages in spleen
digest membrane bits
separate heme from globin
hydrolyze globin (amino acids)
remove iron from heme
convert heme to biliverdin
convert biliverdin to bilirubin
becomes bile product in feces
Erythrocyte Disorders
Polycythemia is an excess of RBC
primary polycythemia is due to cancer of
erythropoietic cell line in the red bone marrow
RBC count as high as 11 million/L; hematocrit of 80%
secondary polycythemia from dehydration,
emphysema, high altitude, or physical conditioning
RBC count only up to 8 million/L
Dangers of polycythemia
increased blood volume, pressure and viscosity can
lead to embolism, stroke or heart failure
Anemia - Deficiency of RBCs or Hb
Causes of anemia
inadequate erythropoiesis or hemoglobin synthesis
inadequate vitamin B12 (cobalamin) or folate (B9, a derivative of B12)
from poor nutrition
lack of intrinsic factor due to autoimmune destruction of parietal cells
(pernicious anemia)
iron-deficiency anemia
kidney failure & insufficient erythropoietin hormone
aplastic anemia is complete cessation (cause unknown)
hemorrhagic anemias from loss of blood
hemolytic anemias from RBC destruction
Effects of anemia
tissue hypoxia and necrosis (short of breath & lethargic)
low blood osmolarity (tissue edema)
low blood viscosity (heart races & pressure drops)
Sickle-Cell Disease & Thalassemia
Sickle-Cell is hereditary Hb defect of African Americans
autosomal recessive allele modifies hemoglobin structure
homozygous recessive for HbS have sickle-cell disease
heterozygous recessive for HbS have sickle-cell trait
sickle-cell disease individual has shortened life
HbS turns to gel in low oxygen concentrations, agglutinating cells and
blocking vessels
intense pain, heart failure, paralysis, anemia, hypoxemia
HbS is indigestible to malaria parasites so gene persists despite
its harmful effects to the homozygous individuals
Thalassemia is hereditary Hb defect seen among people
of Mediterranean area
autosomal recessive
deficiency or absence of alpha or beta hemoglobin
RBC counts less than 2 million cells/L
Blood Types
RBC antigens
called agglutinogens A & B
inherited combinations of
proteins, glycoproteins and
glycolipids on red blood cell
Plasma antibodies
called agglutinins anti-A & -B
gamma globulins in blood
plasma that recognize (stick to)
foreign agglutinogens on RBCs
responsible for RBC agglutination in mismatched
blood transfusions
The ABO Group
ABO blood type is determined by presence or absence
of agglutinogens A & B on RBCs
blood type A person has A agglutinogens, blood type B person has
B agglutinogens, AB has both & blood type O has neither
blood type O is the most common; AB the rarest
Agglutinins appear in the plasma 2-8 months after birth &
at maximum concentration at 10 yr.
agglutinins A and/or B, both or none are in plasma
you do not have those that would react against your own
agglutinogens
each agglutinin, usually IgM, can attach to several agglutinogens
at the same time causing agglutination (clumping)
Agglutination of Erythrocytes
Transfusions
Donor
Reciepient
O A B AB
O x
A x x
B x x
AB x x x x
Mismatched Transfusion Reaction
Agglutinated RBCs
block blood vessels
& rupture
free Hb can block kidney
tubules & cause death
Concept of universal
donors and recipients is obsolete
AB was called universal recipient since it lacks both
antibody A and B; O was called universal donor
problem was donors plasma could have antibodies
solution is giving packed cells with minimum plasma
The Rh Group
Rh or D agglutinogens discovered in rhesus monkey in 1940
blood type is Rh+ if agglutinogens present on RBCs
Rh frequencies vary among ethnic groups
Anti-D agglutinins are not normally present in blood
form only in individuals exposed to Rh+ blood
Rh- pregnant woman carrying an Rh+ fetus or blood transfusion of Rh+
blood
no problems result with either the first transfusion or the first pregnancy
2
nd
time: abortion or miscarriage
hemolytic disease of the newborn (erythroblastosis fetalis) occurs if mother has
formed antibodies & is pregnant with 2nd Rh+ child
RhoGAM is given to pregnant woman to prevent antibody
formation and prevent any future problems
RhoGAM binds fetal agglutinogens in her blood so she will not form
antibodies against them during the pregnancy
Hemolytic Disease of Newborn
Mothers antibodies attack fetal blood causing severe anemia
& toxic brain syndrome from excessive bilirubin in blood
treatment is phototherapy to degrade bilirubin or exchange
transfusion to completely replace infants blood
Leukocyte Descriptions (WBCs)
Granulocytes
eosinophils - pink-orange granules & bilobed nucleus (2-4%)
basophils - abundant, dark violet granules (<1%)
large U- to S-shaped nucleus hidden by granules
neutrophils - multilobed nucleus (60-70%)
fine reddish to violet granules in cytoplasm
Agranulocytes
lymphocytes - round, uniform dark violet nucleus (25-33%)
variable amounts of bluish cytoplasm (scanty to abundant)
monocytes - kidney- or horseshoe-shaped nucleus (3-8%)
large cell with abundant cytoplasm
Granulocyte Functions
Neutrophils
in bacterial infections
most abundant leukocyte
phagocytosis of bacteria
releases antimicrobial chemicals
Eosinophils
in parasitic infections or allergies
phagocytosis of antigen-antibody complexes, allergens &
inflammatory chemicals
release enzymes destroy parasites such as worms
Basophils
in allergic reactions (anaphylaxis) and inflammation
cooperate with mast cells
secrete histamine (vasodilator)
secrete heparin (anticoagulant)
Agranulocyte Functions
Lymphocytes
in diverse infections & immune responses
destroy cancer & foreign cells & virally infected cells
present antigens to activate other immune cells
coordinate actions of other immune cells
secrete antibodies & provide immune memory
Monocytes
in viral infections & inflammation
differentiate into macrophages
phagocytize pathogens and debris
present antigens to activate other immune cells
Abnormalities of Leukocyte Count
Leukopenia = low WBC count (<5000/L)
causes -- radiation, poisons, infectious disease
effects -- elevated risk of infection
Leukocytosis = high WBC count (>10,000/L)
causes -- infection, allergy & disease
differential count -- distinguishes % of each cell type
Leukemia = cancer of hemopoietic tissue
myeloid and lymphoid -- uncontrolled WBC production
acute and chronic -- death in either months or 3 years
effects -- normal cell % disrupted, patient subject to
opportunistic infection, anemia & impaired clotting
Hemostasis - The Control of Bleeding
Effective at closing breaks in small vessels
3 hemostatic mechanisms all involve platelets
Platelets
Small fragments of megakaryocyte cytoplasm
2-4 m diameter & containing granules
pseudopods provide amoeboid movement & phagocytosis
Normal Count -- 130,000 to 400,000 platelets/L
Functions
secrete clotting factors, growth factors for endothelial
repair, and vasoconstrictors in broken vessels
form temporary platelet plugs
dissolve old blood clots
phagocytize bacteria
attract WBCs to sites of inflammation
Vascular Spasm
Prompt constriction of a broken vessel
Triggers for a vascular spasm
some pain receptors directly innervate constrictors
lasts only a few minutes
injury to smooth muscle
longer-lasting constriction
platelets release serotonin, chemical vasoconstrictor
Provides time for other 2 mechanisms to work
Platelet Plug Formation
Normal endothelium very smooth & coated with
prostacyclin (platelet repellent)
Broken vessel exposes rough surfaces of collagen
Platelet plug formation begins
platelet pseudopods stick to damaged vessel and other platelets --
pseudopods contract and draw walls of vessel together forming a
platelet plug
platelets degranulate releasing a variety of substances
serotonin is a vasoconstrictor
adenosine diphosphate (ADP) attracts & degranulates more platelets
thromboxane A2, an eicosanoid that promotes aggregation,
degranulation & vasoconstriction
Positive feedback cycle is active until break in vessel is
sealed
Coagulation
Clotting is the most effective defense against bleeding ---
needs to be quick but accurate
conversion of plasma protein fibrinogen into insoluble
fibrin threads which form framework of clot
Procoagulants or clotting factors (inactive form produced
by the liver) are present in the plasma
activate one factor and it will activate the next to form a
reaction cascade
Factors released by the tissues cause the extrinsic
cascade pathway to begin (damaged vessels)
Factors found only in the blood itself cause the intrinsic
cascade pathway to begin (platelet degranulation)
Both cascades normally occur together
Coagulation Pathways
Extrinsic pathway
initiated by tissue
thromboplastin
cascade from factor
VII to V to X
Intrinsic pathway
initiated by factor XII
cascade from factor XI
to IX to VIII to X
Calcium is required
for either pathway
15 seconds 3-6 minutes
Enzyme Amplification in Clotting
Rapid clotting occurs since each activated enzyme
produces a large number of enzyme molecules in the
following step.
Completion of Coagulation
Coagulation is completed because of the formation
of enzymes in a stepwise fashion
Factor X produces prothrombin activator
Prothrombin activator
converts prothrombin
to thrombin
Thrombin converts
fibrinogen into fibrin
Positive feedback occurs as thrombin speeds up the
formation of prothrombin activator
The Fate of Blood Clots
Clot retraction occurs within 30 minutes
pseudopods of platelets contract condensing the clot
Platelet-derived growth factor is secreted by
platelets & endothelial cells
mitotic stimulant for fibroblasts and smooth muscle to
multiply & repair the damaged vessel
Fibrinolysis or dissolution of a clot
factor XII speeds up the formation of kallikrein enzyme
kallikrein converts plasminogen into plasmin, a
fibrin-dissolving enzyme or clot buster
Blood Clot Dissolution
Positive feedback occurs
Plasmin promotes formation of kallikrein
Positive
Feedback
Prevention of Inappropriate Coagulation
Platelet repulsion
platelets do not adhere to prostacyclin-coating
Thrombin dilution
normally diluted by rapidly flowing blood
heart slowing in shock can result in clot formation
Natural anticoagulants
antithrombin produced by the liver deactivates
thrombin before it can act on fibrinogen
heparin secreted by basophils & mast cells
interferes with formation of prothrombin activator
Coagulation Disorders
Thrombocytopenia
platelet count below 100,000/L (bruise easily)
Hemophilia
genetic lack of any clotting factor that affects
coagulation
X-linked recessive in males (inherit from mother)
hemophilia A is missing factor VIII (83% of cases)
hemophilia B is missing factor IX (15% of cases)
physical exertion causes bleeding & excruciating pain
transfusion of plasma or purified clotting factors
factor VIII now produced by transgenic bacteria
Coagulation Disorders
Unwanted coagulation
thrombosis = abnormal clotting in unbroken vessel
embolism = unwanted clot traveling in a vessel
Infarction or tissue death may occur if clot
blocks blood supply to an organ (MI or stroke)
650,000 Americans die annually of thromboembolism
Disseminated intravascular coagulation (DIC)
widespread clotting of blood within unbroken vessels
triggered by bacteria (septicemia) or if blood slows
down or stops as in cardiac arrest
Vous aimerez peut-être aussi
- Red Blood Cells, Functions, Diseases A Simple Guide To The Condition, Diagnosis, Treatment, And Related ConditionsD'EverandRed Blood Cells, Functions, Diseases A Simple Guide To The Condition, Diagnosis, Treatment, And Related ConditionsPas encore d'évaluation
- Lecture 2-Blood RBC WBCDocument51 pagesLecture 2-Blood RBC WBCsamayaPas encore d'évaluation
- The Circulatory System: BloodDocument59 pagesThe Circulatory System: BloodKristian Dave DivaPas encore d'évaluation
- Anatomy and Physiology of BLOOD: Preet KaurDocument37 pagesAnatomy and Physiology of BLOOD: Preet KaurPreet KaurPas encore d'évaluation
- BloodDocument80 pagesBloodHem Chandra ShahPas encore d'évaluation
- BloodDocument76 pagesBloodKerby Dela Fuente Alison100% (1)
- CVS BloodDocument39 pagesCVS BloodMohan GuptaPas encore d'évaluation
- BloodDocument49 pagesBloodDale TelgenhoffPas encore d'évaluation
- Case 11Document63 pagesCase 11حكيم العجلانPas encore d'évaluation
- The Circulatory System: BloodDocument69 pagesThe Circulatory System: BloodMozesCuatePas encore d'évaluation
- BLOOD LecDocument46 pagesBLOOD LecGrape JuicePas encore d'évaluation
- AnaemiasDocument60 pagesAnaemiasbvkjtzrvnyPas encore d'évaluation
- 1 BloodDocument77 pages1 BloodVisaNathan100% (1)
- BLOOD Update-1Document56 pagesBLOOD Update-1ahmadfadi343Pas encore d'évaluation
- Blood Cells, Immunity and Blood ClottingDocument65 pagesBlood Cells, Immunity and Blood Clottingmunaamuummee100% (1)
- Blood: Cardiovascular SystemDocument75 pagesBlood: Cardiovascular SystemTina MinottPas encore d'évaluation
- Haemopoiesis: Composition of Whole Blood & Its ComponentsDocument8 pagesHaemopoiesis: Composition of Whole Blood & Its ComponentsSafiya JamesPas encore d'évaluation
- Topics of This Lecture: RBC: - Structural Characteristics - Hemoglobin - Erythropoiesis - Erythrocytes DestructionDocument23 pagesTopics of This Lecture: RBC: - Structural Characteristics - Hemoglobin - Erythropoiesis - Erythrocytes DestructionHashim GhazoPas encore d'évaluation
- Hematologic DisordersDocument30 pagesHematologic DisordersUday Kumar100% (1)
- PSG 712 Blood-1Document54 pagesPSG 712 Blood-1preciousPas encore d'évaluation
- Hematology 2020Document63 pagesHematology 2020odiodi57Pas encore d'évaluation
- Body Fluids and Their Circulation 1Document96 pagesBody Fluids and Their Circulation 1sanmathiPas encore d'évaluation
- Blood: - Hematopoiesis (Hemopoiesis) : Blood CellDocument5 pagesBlood: - Hematopoiesis (Hemopoiesis) : Blood Cellflay1618Pas encore d'évaluation
- Physiology of Blood: Ratna Kusumawati Department of Physiology, Faculty of Medicine Universitas Sebelas Maret SurakartaDocument60 pagesPhysiology of Blood: Ratna Kusumawati Department of Physiology, Faculty of Medicine Universitas Sebelas Maret SurakartaZidanePas encore d'évaluation
- Physiology of Blood: Ratna Kusumawati Department of Physiology, Faculty of Medicine Universitas Sebelas Maret SurakartaDocument65 pagesPhysiology of Blood: Ratna Kusumawati Department of Physiology, Faculty of Medicine Universitas Sebelas Maret SurakartaRizal AbdurrahmanPas encore d'évaluation
- Functions of BloodDocument12 pagesFunctions of BloodAngieRamirezPas encore d'évaluation
- Blood: - The Only Fluid Tissue in The Human Body - Classified As A Connective TissueDocument39 pagesBlood: - The Only Fluid Tissue in The Human Body - Classified As A Connective TissueJorPas encore d'évaluation
- 1-Blood Composition and FunctionDocument43 pages1-Blood Composition and FunctionKaleem Mohammad100% (2)
- Blood: - Enzymes, E.G. Certain Clotting FactorsDocument14 pagesBlood: - Enzymes, E.G. Certain Clotting FactorsDerrick kinyaPas encore d'évaluation
- Introduction To HematologyDocument95 pagesIntroduction To HematologyAhmad Farhan Hassan0% (1)
- Chapter 4 Blood Coagulation and Coagulation DisordersDocument27 pagesChapter 4 Blood Coagulation and Coagulation DisordersdaisysintszwaiPas encore d'évaluation
- Red Blood Cells, Anemia, and PolycythemiaDocument7 pagesRed Blood Cells, Anemia, and PolycythemiaShi no Me100% (1)
- LEC3Document26 pagesLEC3Jessica StewartPas encore d'évaluation
- Blood: Group 1Document25 pagesBlood: Group 1Arian May MarcosPas encore d'évaluation
- Biology Part-1 NotesDocument59 pagesBiology Part-1 NotesgjbxPas encore d'évaluation
- Physio. D. Suroor. L1. BloodDocument24 pagesPhysio. D. Suroor. L1. Bloodزين العابدين محمد عويشPas encore d'évaluation
- RBC DISORDERS Part I VR 2022Document63 pagesRBC DISORDERS Part I VR 2022victoria tavasPas encore d'évaluation
- Module 9 - 4 - Circulatory SystemDocument34 pagesModule 9 - 4 - Circulatory SystemThea Shaine B. SILARDEPas encore d'évaluation
- Fisiologi Cairan Tubuh & Darah: Dr. Nindya Aryanty, M.Med - EdDocument43 pagesFisiologi Cairan Tubuh & Darah: Dr. Nindya Aryanty, M.Med - EdIman TaufiqPas encore d'évaluation
- Blood Lecture PowerpointDocument116 pagesBlood Lecture Powerpointdrmarybeth100% (3)
- Nurs 107 Blood Physiology Lecture 4Document46 pagesNurs 107 Blood Physiology Lecture 4Ann AgyeiwaaPas encore d'évaluation
- Blood PDFDocument55 pagesBlood PDFlorrainebarandonPas encore d'évaluation
- PHS 221 1Document44 pagesPHS 221 1metasynthronos748Pas encore d'évaluation
- Blood Physiology-Lecture 4Document54 pagesBlood Physiology-Lecture 4alfaristurkiPas encore d'évaluation
- Hematopoiesis and ErytherokiniticsDocument20 pagesHematopoiesis and ErytherokiniticsShahid F.S.D.Pas encore d'évaluation
- Introd., Haemopoiesis & Plasma ProteinsDocument119 pagesIntrod., Haemopoiesis & Plasma ProteinsShamsuddeen UsmanPas encore d'évaluation
- 6.2 Human BloodDocument48 pages6.2 Human BloodSmart ThemisPas encore d'évaluation
- Blood PhysiologyDocument8 pagesBlood PhysiologypjcaneroPas encore d'évaluation
- Blood and Blood ComponentsDocument19 pagesBlood and Blood ComponentsMiljun CatacataPas encore d'évaluation
- University of Guyana Health Sciences FacultyDocument44 pagesUniversity of Guyana Health Sciences FacultySatrohan SurjnarinePas encore d'évaluation
- Lecture 9 CVSDocument118 pagesLecture 9 CVSMuhammad Abbas WaliPas encore d'évaluation
- Cardiovascular Physiology 4Document75 pagesCardiovascular Physiology 4maxmus4Pas encore d'évaluation
- Haemopoetic SystemDocument73 pagesHaemopoetic SystemManjunathPas encore d'évaluation
- Blood Lectures 2014 PIO 205Document84 pagesBlood Lectures 2014 PIO 205Philip Abayomi VincentPas encore d'évaluation
- Ch18 BloodDocument31 pagesCh18 BloodNoah DominoPas encore d'évaluation
- RBC DISORDERS StudentsDocument84 pagesRBC DISORDERS Studentskimberly abianPas encore d'évaluation
- Avian Physiology Lecture 003Document36 pagesAvian Physiology Lecture 003Susai Mari NixonPas encore d'évaluation
- Blood Part 1Document35 pagesBlood Part 1Keziah TampusPas encore d'évaluation
- Fast Facts: Deficiencia de piruvato quinasa para pacientes y familiares: Una enfermedad genética rara que afecta a los glóbulos rojos Información + Asumir el control = El mejor resultadoD'EverandFast Facts: Deficiencia de piruvato quinasa para pacientes y familiares: Una enfermedad genética rara que afecta a los glóbulos rojos Información + Asumir el control = El mejor resultadoPas encore d'évaluation
- Study of Microscopic Anatomy - Only 200 Different Cells Types - Four Primary Tissue ClassesDocument61 pagesStudy of Microscopic Anatomy - Only 200 Different Cells Types - Four Primary Tissue ClassesvanderphysPas encore d'évaluation
- Without This Message by Purchasing Novapdf : Print To PDFDocument1 pageWithout This Message by Purchasing Novapdf : Print To PDFvanderphysPas encore d'évaluation
- Cells Capable of Shortening & Converting The Chemical Energy of ATP Into Mechanical Energy - Types of Muscle - Physiology of Skeletal MuscleDocument64 pagesCells Capable of Shortening & Converting The Chemical Energy of ATP Into Mechanical Energy - Types of Muscle - Physiology of Skeletal MusclevanderphysPas encore d'évaluation
- Study of Microscopic Anatomy - Only 200 Different Cells Types - Four Primary Tissue ClassesDocument61 pagesStudy of Microscopic Anatomy - Only 200 Different Cells Types - Four Primary Tissue ClassesvanderphysPas encore d'évaluation
- 16 Sense OrgansDocument79 pages16 Sense OrgansvanderphysPas encore d'évaluation
- 9 Skeletal SystemDocument63 pages9 Skeletal Systemvanderphys100% (1)
- Nucleus and Nucleic Acids - Protein Synthesis and Secretion - DNA Replication and The Cell Cycle - Chromosomes and HeredityDocument53 pagesNucleus and Nucleic Acids - Protein Synthesis and Secretion - DNA Replication and The Cell Cycle - Chromosomes and HeredityvanderphysPas encore d'évaluation
- 25 Digestive SystemDocument79 pages25 Digestive SystemvanderphysPas encore d'évaluation
- 14 The Central Nervous SystemDocument61 pages14 The Central Nervous SystemvanderphysPas encore d'évaluation
- 7 Integumentary SystemDocument38 pages7 Integumentary Systemvanderphys100% (1)
- 8 Bone TissueDocument39 pages8 Bone Tissuevanderphys100% (1)
- 22 Respiratory SystemDocument99 pages22 Respiratory SystemvanderphysPas encore d'évaluation
- 600 Human Skeletal Muscles - General Structural & Functional OrganizationDocument61 pages600 Human Skeletal Muscles - General Structural & Functional OrganizationvanderphysPas encore d'évaluation
- 25 Digestive SystemDocument79 pages25 Digestive SystemvanderphysPas encore d'évaluation
- BPT First Year Syllabus Rabindranath Tagore UniversityDocument33 pagesBPT First Year Syllabus Rabindranath Tagore Universitysubhu7678Pas encore d'évaluation
- Nursing Care of A Child With FractureDocument40 pagesNursing Care of A Child With FractureSomyee Pachuau100% (2)
- Inflammation and RepairDocument38 pagesInflammation and RepairIsaac Nsiah Acheampong100% (1)
- Health and Physical Assessment in Nursing 3rd Edition DAmico Test Bank DownloadDocument27 pagesHealth and Physical Assessment in Nursing 3rd Edition DAmico Test Bank DownloadSharon Reper100% (24)
- Giraffe Blood CirculationDocument9 pagesGiraffe Blood Circulationthalita asriandinaPas encore d'évaluation
- Anatomy of PalateDocument33 pagesAnatomy of Palatekvellingiri0% (1)
- Lesson 2 The Cell: Prepared By: Asley Raven MacatiagDocument29 pagesLesson 2 The Cell: Prepared By: Asley Raven MacatiagescribdeePas encore d'évaluation
- Multiple PregnancyDocument71 pagesMultiple PregnancyAndre PutraPas encore d'évaluation
- Urine Therapy by Flora Peschek Böhmer 1 PDFDocument160 pagesUrine Therapy by Flora Peschek Böhmer 1 PDFAndy Ochumba100% (8)
- Cranial Nerve: Dr. Dian Prasetyo Wibisono, M.SCDocument49 pagesCranial Nerve: Dr. Dian Prasetyo Wibisono, M.SCgenePas encore d'évaluation
- Biology Project: C XIBDocument18 pagesBiology Project: C XIBPranjal SharmaPas encore d'évaluation
- Chapter 24 (Digestive System) Chapter 24 (Digestive System)Document30 pagesChapter 24 (Digestive System) Chapter 24 (Digestive System)Pranali BasuPas encore d'évaluation
- 1.4 Plant Structure and FunctionsDocument15 pages1.4 Plant Structure and Functionsaddy prairiePas encore d'évaluation
- A.1.1 Embriologi OtakDocument28 pagesA.1.1 Embriologi OtakoliviaPas encore d'évaluation
- Michael Jackson Full Autopsy 2Document149 pagesMichael Jackson Full Autopsy 2nadia box50% (2)
- Normal ChestDocument82 pagesNormal ChestMonica CherladyPas encore d'évaluation
- Health Assessment Semi Finals NotesDocument43 pagesHealth Assessment Semi Finals NotesDazell VarronPas encore d'évaluation
- Chapter - 5 Fundamental Unit of LifeDocument12 pagesChapter - 5 Fundamental Unit of LifehelloPas encore d'évaluation
- BTEC Sport Level 3 Revision Guide Muscular SystemDocument19 pagesBTEC Sport Level 3 Revision Guide Muscular Systemkanuku100% (1)
- Wound Repair and RegenrationDocument9 pagesWound Repair and RegenrationShivanshu SiyanwalPas encore d'évaluation
- PBSDocument72 pagesPBSKavi KaviPas encore d'évaluation
- Disseminated Intravascular CoagulationDocument37 pagesDisseminated Intravascular CoagulationhipoclaudioPas encore d'évaluation
- MFM42Support - Answers of End Module MSK1Document8 pagesMFM42Support - Answers of End Module MSK1Mohammed HashemPas encore d'évaluation
- 5-Locomotion and Movement PDFDocument4 pages5-Locomotion and Movement PDFPraveen KumarPas encore d'évaluation
- Surgical Anatomy of The Biliary TractDocument5 pagesSurgical Anatomy of The Biliary TracthoangducnamPas encore d'évaluation
- Dr. Witra Irfan, SP.B (K) V - DVTDocument29 pagesDr. Witra Irfan, SP.B (K) V - DVTmuhammad azharanPas encore d'évaluation
- Endocrine System Worksheet AnswersDocument3 pagesEndocrine System Worksheet AnswersmariaPas encore d'évaluation
- 02 IVD Hernia Case Report MedBac&IEDocument18 pages02 IVD Hernia Case Report MedBac&IEClaude ReyesPas encore d'évaluation
- Systematic "A" Level Biology. Basic and Simplified Revision NotesDocument458 pagesSystematic "A" Level Biology. Basic and Simplified Revision NotesNelima Stella mercy100% (2)
- Nervous Tissue System-92618Document151 pagesNervous Tissue System-92618gokulkrishnayadhavPas encore d'évaluation