Académique Documents
Professionnel Documents
Culture Documents
Acid Base Equilibria
Transféré par
gkawsar22Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Acid Base Equilibria
Transféré par
gkawsar22Droits d'auteur :
Formats disponibles
Acid-base equilibria
Introduction:
It is essential that you can write
the expression for K
a
of a weak
acid.
Make sure that you know how to
use your calculator to evaluate
logarithms, and how to turn p
H
and
pK
a
values into [H
+
] and K
a
values.
Acid-base equilibria
Buffer solutions do not have a
constant p
H
. They resist changes
in p
H
.
You may use H
+
, H
+
(aq) or H
3
O
+
as the formula of hydrogen ion.
Things to learn
Arrhenius acid is a substance that
produce H
+
ions when they dissolve in
water:
HA(aq) H
+
(aq) + A
-
(aq)
Arrhenius base is a substance that produce
the hydroxide (OH-) ion in solution:
B(aq) + H
2
O(aq) BH
+
(aq) + OH
-
(aq)
It is suggested that the H
+
ion exists in association with a
water molecule as the H
3
O
+
ion, called the oxonium ion.
Things to learn
Things to learn
Things to learn
p
OH
= - log[OH
-
(aq)]
p
H
+ p
OH
= 14 at 25
o
C.
A strong acid is totally ionized in
aqueous solution, and a weak acid is
only partially ionized in aqueous
solution.
K
a
, for a weak acid, HA = [H
+
][A
-
] / [HA].
p
K
a = - log K
a
Conjugate acid/base pairs
Conjugate acid/base pairs
P
H
p
H
Scale
p
H
Scale
p
H
of strong acids and bases
So at neutral solution is one where
[H
+
] = [OH
-
], and the p
H
= 7
An acid solution is one where [H
+
] >
[OH
-
], and the p
H
< 7.
An alkaline solution is one where [H
+
]
< [OH
-
], and the p
H
> 7.
p
H
of strong acids and bases
For a strong monobasic acid:
p
H
= - log [acid]
Therefore the p
H
of a 0.321 mol dm
-3
solution of HCl = - log (0.0321) = 0.49
For a strong alkali with one OH
-
ion in
the formula:
p
OH
= - log[alkali] and
p
H
= 14 p
OH
p
H
of strong acids and bases
Therefore the p
H
of a 0.109 mol dm
-3
solution of NaOH is calculated:
p
OH
= -log(0.109) = 0.96
Therefore p
H
= 14 0.96 = 13.04
p
H
of strong acids and bases
Therefore the p
H
of a 0.109 mol dm
-3
solution of NaOH is calculated:
p
OH
= -log(0.109) = 0.96
Therefore p
H
= 14 0.96 = 13.04
p
H
of weak acids
Weak acids are partially ionized. A
general equation for this is:
HA(aq) = H
+
(aq) + A
-
(aq)
The acid produces equal amounts of H+
and A-, so that:
[H
+
(aq) = [A
-
(aq)]
Worked example
Indicators
For use in a titration, an indicator must
change colour within the pH of the vertical
part of the titration curve.
Vertical range of pH Suitable indicator
Strong acid and base
Weak acid/strong base
Strong acid/weak base
3.5 to 10.5
7 to 10.5
3.5 to 7
Methyl orange or
phenolphthalein
Phenolphthalein
Methyl orange
Buffer solutions
Buffer solutions
The weak acid is partially ionized:
CH
3
COOH(aq) H
+
(aq) + CH
3
COO
-
(aq)
The CH
3
COO
-
ions from the salt suppress
most of the ionization of the acid, and so
both [CH
3
COOH(aq)] and [CH
3
COO
-
(aq)]
are large.
Buffer solutions
Calculation of the pH of a buffer solution
Calculation of the pH of a buffer solution
Worked example
Enthalpy of neutralization
Checklist
For attempting the questions on this topic, check
that you:
Can identify acid/base conjugate pairs.
Can define pH and K
w
.
Can define K
a
and pK
a
for weak acids.
Understand what is meant by the terms strong
and weak as applied to acids and bases.
Can calculate the pH of solutions of strong
acids, strong bases and weak acids.
Checklist
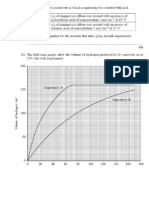
Can recall the titration curves for the
neutralization of strong and weak acids.
Can use the curves to calculate the value
of K
a
for a weak acid.
Understand the reasons for the choice of
indicator in an acid/base titrations.
Can define a buffer solution, explain its
mode of action and calculate its p
H
.
Test your knowledge and understanding
For the following questions, cover the
margin, write your answer, then check to
see if you are correct.
Identify the acid/base conjugate pairs in
the reaction:
H
2
SO
4
+ CH
3
COOH CH
3
COOH
2
+
+
HSO
4
-
Test your knowledge and understanding
Calculate the p
H
of :
0.11 mol dm
-3
HCl
0.11 mol dm
-3
LiOH
0.11 mol dm
-3
Ba(OH)
2
Calculate the p
H
of 0.22 mol dm
-3
C
2
H
5
COOH which has pK
a
= 4.87.
Vous aimerez peut-être aussi
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5794)
- Qualifying Test Result (2014-15) Subject: Chemistry: Bangladesh School MuscatDocument2 pagesQualifying Test Result (2014-15) Subject: Chemistry: Bangladesh School Muscatgkawsar22Pas encore d'évaluation
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (399)
- Report of Preparatory Mockigcse SCDocument55 pagesReport of Preparatory Mockigcse SCgkawsar22Pas encore d'évaluation
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- ElectrolysisDocument7 pagesElectrolysisgkawsar22Pas encore d'évaluation
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (894)
- AtomsDocument4 pagesAtomsgkawsar22Pas encore d'évaluation
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- Periodic TableDocument3 pagesPeriodic Tablegkawsar22Pas encore d'évaluation
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- Test Nichrome Wire Wire Wire ColourDocument2 pagesTest Nichrome Wire Wire Wire Colourgkawsar22Pas encore d'évaluation
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- Relative Atomic MassDocument8 pagesRelative Atomic Massgkawsar22Pas encore d'évaluation
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- Types of ExtractionDocument5 pagesTypes of ExtractionMohammad NafaiPas encore d'évaluation
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (587)
- Acid & BaseDocument3 pagesAcid & Basegkawsar22Pas encore d'évaluation
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (265)
- Energy From ChemicalsDocument4 pagesEnergy From Chemicalsgkawsar22Pas encore d'évaluation
- BondingDocument5 pagesBondinggkawsar22Pas encore d'évaluation
- Crude OilDocument4 pagesCrude Oilgkawsar22Pas encore d'évaluation
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- RadiationDocument3 pagesRadiationgkawsar22Pas encore d'évaluation
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (73)
- Acid & BaseDocument3 pagesAcid & Basegkawsar22Pas encore d'évaluation
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (344)
- The Aqueous Chemistry of CationsDocument11 pagesThe Aqueous Chemistry of Cationsgkawsar22Pas encore d'évaluation
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- Classifying Materials Activity 5.1 - Physical Properties TestDocument1 pageClassifying Materials Activity 5.1 - Physical Properties Testgkawsar22Pas encore d'évaluation
- Activity 13.3: How Does Concentration Affect Reaction Rate?Document2 pagesActivity 13.3: How Does Concentration Affect Reaction Rate?gkawsar22Pas encore d'évaluation
- HeinemannIGCSE Chemistry Chapter1Document3 pagesHeinemannIGCSE Chemistry Chapter1gkawsar22Pas encore d'évaluation
- GCSE CHEMISTRY ACIDS, BASES & SALTS HIGH DEMAND QUESTIONSDocument21 pagesGCSE CHEMISTRY ACIDS, BASES & SALTS HIGH DEMAND QUESTIONSeeenusPas encore d'évaluation
- 0906 O Level Grade BoundariesDocument2 pages0906 O Level Grade Boundariesryuzaki589Pas encore d'évaluation
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- Q 2Document25 pagesQ 2gkawsar22Pas encore d'évaluation
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2219)
- A Guide To Practical QuestionsDocument10 pagesA Guide To Practical Questionsgkawsar22Pas encore d'évaluation
- Making SaltsDocument1 pageMaking Saltsgkawsar22Pas encore d'évaluation
- Seperatting and AnalysisDocument3 pagesSeperatting and Analysisgkawsar22Pas encore d'évaluation
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1090)
- Q 1Document36 pagesQ 1gkawsar22Pas encore d'évaluation
- Introducing Energy Changes in ReactionsDocument2 pagesIntroducing Energy Changes in Reactionsgkawsar22Pas encore d'évaluation
- Rate of ReactionDocument3 pagesRate of Reactiongkawsar22Pas encore d'évaluation
- Oxygen and OxidesDocument5 pagesOxygen and Oxidesgkawsar22Pas encore d'évaluation
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (119)
- Kinetic Theory of DiffusionDocument1 pageKinetic Theory of Diffusiongkawsar22Pas encore d'évaluation
- Introducing Reversible ReactionsDocument3 pagesIntroducing Reversible Reactionsgkawsar22Pas encore d'évaluation
- Roadblocks Overcome Cruise PurchaseTITLE Top 15 Cruise Hesitations Answered TITLE How to Convince People Cruises Worth CostDocument4 pagesRoadblocks Overcome Cruise PurchaseTITLE Top 15 Cruise Hesitations Answered TITLE How to Convince People Cruises Worth CostJanel Castillo Balbiran33% (3)
- General Specifications: Detail ADocument1 pageGeneral Specifications: Detail AJeniel PascualPas encore d'évaluation
- Quiz EmbryologyDocument41 pagesQuiz EmbryologyMedShare90% (67)
- Placenta Previa Case StudyDocument59 pagesPlacenta Previa Case StudySiergs Smith GervacioPas encore d'évaluation
- Nicenstripy Gardening Risk AssessmentDocument38 pagesNicenstripy Gardening Risk AssessmentVirta Nisa100% (1)
- Very Easy Toeic Units 7 - 12 (Q1)Document39 pagesVery Easy Toeic Units 7 - 12 (Q1)Minh KhaiPas encore d'évaluation
- Impact of Energy Consumption On The EnvironmentDocument9 pagesImpact of Energy Consumption On The Environmentadawiyah sofiPas encore d'évaluation
- Nitric OxideDocument20 pagesNitric OxideGanesh V GaonkarPas encore d'évaluation
- New930e-4se Ceam031503 930e4se Omm A31937 Up PDFDocument273 pagesNew930e-4se Ceam031503 930e4se Omm A31937 Up PDFSergelen SakhyabazarPas encore d'évaluation
- SCE Research Paper PDFDocument12 pagesSCE Research Paper PDFmuoi2002Pas encore d'évaluation
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)
- Neurons and Nerve Impulses: Nandika Arora and Risa Gaikwad (11 G2)Document17 pagesNeurons and Nerve Impulses: Nandika Arora and Risa Gaikwad (11 G2)RisaPas encore d'évaluation
- HTM 2025 2 (New) Ventilation in HospitalsDocument123 pagesHTM 2025 2 (New) Ventilation in HospitalsArvish RamseebaluckPas encore d'évaluation
- Request For Review FormDocument11 pagesRequest For Review FormJoel MillerPas encore d'évaluation
- FinalsDocument8 pagesFinalsDumpPas encore d'évaluation
- Quality ImprovementDocument3 pagesQuality ImprovementViky SinghPas encore d'évaluation
- Parasitology Lecture Hosts, Symbiosis & TransmissionDocument10 pagesParasitology Lecture Hosts, Symbiosis & TransmissionPatricia Ann JosePas encore d'évaluation
- DR - Hawary Revision TableDocument3 pagesDR - Hawary Revision TableAseel ALshareefPas encore d'évaluation
- EcoLettsandSOM, Dulvy Et Al 2004Document25 pagesEcoLettsandSOM, Dulvy Et Al 2004Nestor TorresPas encore d'évaluation
- Arp0108 2018Document75 pagesArp0108 2018justin.kochPas encore d'évaluation
- Ethamem-G1: Turn-Key Distillery Plant Enhancement With High Efficiency and Low Opex Ethamem TechonologyDocument25 pagesEthamem-G1: Turn-Key Distillery Plant Enhancement With High Efficiency and Low Opex Ethamem TechonologyNikhilPas encore d'évaluation
- Reach Out and Read Georgia Selected For AJC Peachtree Road Race Charity Partner ProgramDocument2 pagesReach Out and Read Georgia Selected For AJC Peachtree Road Race Charity Partner ProgramPR.comPas encore d'évaluation
- Benefits and Limitations of Vojta ApproachDocument50 pagesBenefits and Limitations of Vojta ApproachAlice Teodorescu100% (3)
- Bentel J408Document64 pagesBentel J408Bojan MarkovicPas encore d'évaluation
- Iso 28000Document11 pagesIso 28000Aida FatmawatiPas encore d'évaluation
- Book 1Document94 pagesBook 1JOHNPas encore d'évaluation
- Practical Examination Marking Guideline Grade 12 Physical Science 2019 PDFDocument5 pagesPractical Examination Marking Guideline Grade 12 Physical Science 2019 PDFWonder Bee Nzama100% (1)
- GTT Module 5Document156 pagesGTT Module 5ABDULRAHIMAN RAJEKHANPas encore d'évaluation
- 559 Fault CodeDocument4 pages559 Fault Codeabdelbagi ibrahim100% (1)
- Nutrition During PregnancyDocument8 pagesNutrition During PregnancyHalliahPas encore d'évaluation
- Montgomery County Ten Year Comprehensive Water Supply and Sewerage Systems Plan (2003)Document228 pagesMontgomery County Ten Year Comprehensive Water Supply and Sewerage Systems Plan (2003)rebolavPas encore d'évaluation