Académique Documents
Professionnel Documents
Culture Documents
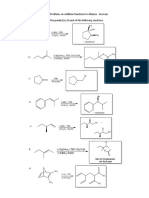
Claisen Condensation, Acetoacetic Ester and Malonic Ester Synthesis
Transféré par
Iqbal YeahTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Claisen Condensation, Acetoacetic Ester and Malonic Ester Synthesis
Transféré par
Iqbal YeahDroits d'auteur :
Formats disponibles
Chapter 19
Synthesis and Reactions of
-Dicarbonyl Compounds: More
Chemistry of Enolate Anions
Introduction
-Dicarbonyl compounds have two carbonyl groups separated by a
carbon
Protons on the -carbon of -dicarbonyl compounds are acidic
(pKa = 9-10)
The acidity can be explained by resonance stabilization of the corresponding
enolate by two carbonyl groups
Chapter19
-Dicarbonyl compounds can be synthesized by the Claisen
condensation
The acetoacetic ester and malonic acid syntheses usedicarbonyl compounds for carbon-carbon bond forming reactions
The acetoacetic ester and malonic ester syntheses usually
conclude with decarboxylation of a -keto acid
Chapter19
The Claisen Condensation: Synthesis of -Keto
Esters
Ethyl acetate undergoes a Claisen condensation when treated with
sodium ethoxide
The product is commonly called an acetoacetic ester
Ethyl pentanoate undergoes an analogous reaction
Chapter19
The overall reaction involves loss of an hydrogen from one ester
and loss of ethoxide from another
The mechanism is an example of the general process of
nucleophilic addition-elimination at an ester carbonyl
Chapter19
Chapter19
The alkoxide base must have the same alkyl group as
the alkoxyl group of the ester
The use of a different alkoxide would result in formation of
some transesterification products
Esters with only one hydrogen do not undergo Claisen
condensation
A second hydrogen on the carbon is necessary so
that it can be deprotonated in Step 3
This deprotonation drives the reaction to completion
Chapter19
Crossed Claisen Condensations
Crossed Claisen condensations can lead to one major product
when one of the two esters has no hydrogen
Chapter19
The Dieckmann condensation is an intramolecular Claisen
condensation
Only 5- and 6-membered rings may be prepared in this way
Chapter19
Introduction
-Dicarbonyl compounds have two carbonyl groups separated by a
carbon
Protons on the -carbon of -dicarbonyl compounds are acidic
(pKa = 9-10)
The acidity can be explained by resonance stabilization of the corresponding
enolate by two carbonyl groups
Chapter19
10
-Dicarbonyl compounds can be synthesized by the Claisen
condensation
The acetoacetic ester and malonic acid syntheses usedicarbonyl compounds for carbon-carbon bond forming reactions
The acetoacetic ester and malonic ester syntheses usually
conclude with decarboxylation of a -keto acid
Chapter19
11
The Acetoacetic Ester Synthesis: Synthesis of
Methyl Ketones (Substituted Acetones)
Alkylation
Alkylation of the enolate derived from acetoacetic ester is called
the acetoacetic ester synthesis
This is an SN2 reaction with the ethyl acetoacetate enolate
acting as the nucleophile
Chapter19
12
A second alkylation can be performed
A stronger base such as potassium tert-butoxide must
be use to deprotonate the monoalkyl ester
Chapter19
13
Hydrolysis of the ester and heating of the resultant -ketoacid
causes decarboxylation
The product is a substituted acetone derivative
Chapter19
14
Example:
Chapter19
15
Ethylacetoacetate serves as a synthetic equivalent of the acetone
enolate
It is possible to use acetone enolate directly, but this would
require a much stronger base and special reaction conditions
If -halo esters are used to alkylate the enolate, -keto acids are
obtained
Chapter19
16
Acetoacetic Ester Dianion: Alkylation at the Terminal
Carbon
Treating acetoacetic ester with two equivalents of a very strong
base produces the dianion
Alkylation of the dianion occurs first at the terminal carbon
The terminal carbanion is more nucleophilic and more basic because it is
stabilized by only one carbonyl group
Chapter19
17
The Malonic Ester Synthesis: Synthesis of
Substituted Acetic Acids
Alkylation of diethylmalonate, hydrolysis of the diester to the dicarboxylic acid, and decarboxylation can be used to synthesize
mono- and disubstituted acetic acids
The mechanism is analogous to that for the acetoacetic ester
synthesis
In step 1 the stabilized anion is formed
Chapter19
18
In step 2 the anion is mono- or dialkylated using SN2 reactions
Chapter19
19
In step 3 the mono- or dialkylated product is hydrolyzed and
decarboxylated
Chapter19
20
Examples
Chapter19
21
Vous aimerez peut-être aussi
- Alpha Hydrogen 090812Document93 pagesAlpha Hydrogen 090812Maisarah HalimPas encore d'évaluation
- Aldehydes and Ketones I NucleophIlic Addition To The Carbonyl GroupDocument41 pagesAldehydes and Ketones I NucleophIlic Addition To The Carbonyl GroupDenisse BadiolaPas encore d'évaluation
- Enol N Ion EnolatDocument39 pagesEnol N Ion EnolatJulia RahayuPas encore d'évaluation
- CARBONYL CONDENSATION REACTIONS 2 (10 Mei 2013)Document34 pagesCARBONYL CONDENSATION REACTIONS 2 (10 Mei 2013)Mammy Nya AllyaPas encore d'évaluation
- Enzymes: A Practical Introduction to Structure, Mechanism, and Data AnalysisD'EverandEnzymes: A Practical Introduction to Structure, Mechanism, and Data AnalysisÉvaluation : 4 sur 5 étoiles4/5 (2)
- tổng hợp nghịchDocument81 pagestổng hợp nghịchruakon_ldt9527100% (1)
- 15 - Aldehyde and KetonesDocument66 pages15 - Aldehyde and KetonesIrfan Raza100% (1)
- Conrad-Limpach Quinoline Synthesis: A. General Description of The ReactionDocument5 pagesConrad-Limpach Quinoline Synthesis: A. General Description of The ReactionLe Tu0% (1)
- Organic Chemistry For USTH Students Lecture 2: Electrophilic Addition To C CDocument107 pagesOrganic Chemistry For USTH Students Lecture 2: Electrophilic Addition To C CminhminhPas encore d'évaluation
- Modern Aldol Reactions, Part2Document346 pagesModern Aldol Reactions, Part2Kybernetikum100% (1)
- Ethylene Oxide Project QuestionDocument1 pageEthylene Oxide Project Questionkaryensam100% (1)
- Perkin ReactionDocument2 pagesPerkin Reactionrajaraghuramvarma100% (1)
- Benzene & Aromatic Compound: Jully Tan School of EngineeringDocument41 pagesBenzene & Aromatic Compound: Jully Tan School of EngineeringSàtz ÑÖÑïtPas encore d'évaluation
- Name Reaction 3569Document38 pagesName Reaction 3569Ashish AmbekarPas encore d'évaluation
- Aromaticity With Huckle's RuleDocument7 pagesAromaticity With Huckle's RuleSk ZPas encore d'évaluation
- CN102807528-PREPARATION METHOD OF 10-Methoxy IminostilbeneDocument4 pagesCN102807528-PREPARATION METHOD OF 10-Methoxy IminostilbeneDipti DodiyaPas encore d'évaluation
- Ugi ReactionDocument11 pagesUgi ReactionNavnath HatvatePas encore d'évaluation
- Oxidative Addition and Reductive Elimination: Peter H.M. BudzelaarDocument23 pagesOxidative Addition and Reductive Elimination: Peter H.M. BudzelaarRana Hassan Tariq100% (1)
- Olefin Polymerization With Ziegler-Natta Catalyst - Chemistry LibreTextsDocument3 pagesOlefin Polymerization With Ziegler-Natta Catalyst - Chemistry LibreTextsasad100% (1)
- Practice Problems On Addition Reactions To Alkenes With AnswersDocument4 pagesPractice Problems On Addition Reactions To Alkenes With AnswersSangetha ChelladoraiPas encore d'évaluation
- Answer KeyDocument6 pagesAnswer KeyMadhavanIcePas encore d'évaluation
- Org Chem Sem 3 Paper 2Document15 pagesOrg Chem Sem 3 Paper 2Rohit DeshmukhPas encore d'évaluation
- Sni, Nighbouring GP Participation & E1cbDocument19 pagesSni, Nighbouring GP Participation & E1cbsaheedvkPas encore d'évaluation
- Witting Reaction by Suman BalyaniDocument22 pagesWitting Reaction by Suman BalyaniSuman Balyani50% (2)
- Schiff and Mannich ReactionsDocument16 pagesSchiff and Mannich ReactionsSat MontesPas encore d'évaluation
- 2013 Group II Lecture Notes (Student) .UnlockedDocument20 pages2013 Group II Lecture Notes (Student) .UnlockedKwok Yee TherPas encore d'évaluation
- Thermodynamics PolymersDocument32 pagesThermodynamics Polymersthuron100% (2)
- HydrocarbonDocument25 pagesHydrocarbonSoham NagPas encore d'évaluation
- Lab ManualDocument19 pagesLab Manualanon_467104036Pas encore d'évaluation
- Benzene and Derivatives Members GroupDocument57 pagesBenzene and Derivatives Members GroupHaris KhanPas encore d'évaluation
- Qualitative Analysis PresentationDocument17 pagesQualitative Analysis PresentationSumeer ShafiqPas encore d'évaluation
- Chapter 18 - Carbonyl CompoundsDocument9 pagesChapter 18 - Carbonyl CompoundsNabindra RuwaliPas encore d'évaluation
- 06 Chapter 1Document63 pages06 Chapter 1Kautsar NurfalaqPas encore d'évaluation
- Retrosynthetic AnalysisDocument3 pagesRetrosynthetic AnalysismmiliyasPas encore d'évaluation
- Huckel Rule of Aromaticity 2 PDFDocument25 pagesHuckel Rule of Aromaticity 2 PDFUmar Farooq100% (1)
- Functional Group InterconversionDocument7 pagesFunctional Group InterconversionSUBHASISH DASHPas encore d'évaluation
- EPOXIDEDocument4 pagesEPOXIDEVidhu PandeyPas encore d'évaluation
- Ib PPT 10 HL PDFDocument38 pagesIb PPT 10 HL PDFzarna nirmal rawalPas encore d'évaluation
- ReagentsDocument5 pagesReagentsSomu Yashawant ChaudhariPas encore d'évaluation
- Carbonyl Compounds 230Document60 pagesCarbonyl Compounds 230mohtasim hasanPas encore d'évaluation
- Multi-Step Organic SynthesisDocument6 pagesMulti-Step Organic SynthesisPhạm Thị Thùy NhiênPas encore d'évaluation
- Nomenclature Sheet 2021,13thDocument89 pagesNomenclature Sheet 2021,13thsane jha vlogsPas encore d'évaluation
- The Sadtler Handbook of Proton NMR Spectra PDFDocument297 pagesThe Sadtler Handbook of Proton NMR Spectra PDFFedegos_toso100% (1)
- Transition Metals H2 QuestionsDocument7 pagesTransition Metals H2 QuestionskitoniumPas encore d'évaluation
- Mitsunobu and Related Reactions - Advances and ApplicationsDocument101 pagesMitsunobu and Related Reactions - Advances and ApplicationsSuresh BabuPas encore d'évaluation
- Alkene and Alkyne - by Resonance PDFDocument45 pagesAlkene and Alkyne - by Resonance PDFPrasad Yarra100% (1)
- Essential Organic Chemistry: Aromaticity: Reactions of Benzene and Substituted BenzenesDocument75 pagesEssential Organic Chemistry: Aromaticity: Reactions of Benzene and Substituted BenzeneschurvaloooPas encore d'évaluation
- Aromatic Compounds and Uses of BenzeneDocument44 pagesAromatic Compounds and Uses of BenzenePaarijat DubeyPas encore d'évaluation
- Hypervalent Iodine: Dess-Martin Periodane: Selective Oxidation of Prim. Alcohols To Aldehydes, Sec. Alcohols To KetonesDocument15 pagesHypervalent Iodine: Dess-Martin Periodane: Selective Oxidation of Prim. Alcohols To Aldehydes, Sec. Alcohols To Ketonesevsgoud_goudPas encore d'évaluation
- Molecular RearrangementsDocument158 pagesMolecular RearrangementsRamesh Katkam75% (4)
- Friedel Crafts ReactionsDocument6 pagesFriedel Crafts ReactionsjanmanchiPas encore d'évaluation
- Disconnection ApproachDocument5 pagesDisconnection ApproachPrashanthPatroPas encore d'évaluation
- Chemistry Alcohols Phenols and Ethers PDFDocument37 pagesChemistry Alcohols Phenols and Ethers PDFMohammed RafiuddinPas encore d'évaluation
- The Diels-Alder Reaction: PROBLEM 22.10Document8 pagesThe Diels-Alder Reaction: PROBLEM 22.10Sandipan Saha100% (1)
- Experiment 3Document3 pagesExperiment 3Madhu KhanPas encore d'évaluation
- Real InDictionary of Chemistry 4 BookDocument33 pagesReal InDictionary of Chemistry 4 Booktire farrokhzadPas encore d'évaluation
- Report PraktikalDocument52 pagesReport PraktikalIqbal Yeah100% (1)
- Mass SpectrometryDocument1 pageMass SpectrometryIqbal YeahPas encore d'évaluation
- Envs4450 9Document59 pagesEnvs4450 9Iqbal YeahPas encore d'évaluation
- PDF Padlet 6 Raman Spectroscopy Sept2014Document22 pagesPDF Padlet 6 Raman Spectroscopy Sept2014Maxvicklye RaynerPas encore d'évaluation
- Magnetic Anisotropy in Nuclear Magnetic Resonance (NMR)Document1 pageMagnetic Anisotropy in Nuclear Magnetic Resonance (NMR)Iqbal YeahPas encore d'évaluation
- PMTDocument12 pagesPMTIqbal YeahPas encore d'évaluation
- AasDocument80 pagesAasIqbal YeahPas encore d'évaluation
- Blank: What Is A "Blank" in Analytical Chemistry?Document7 pagesBlank: What Is A "Blank" in Analytical Chemistry?Iqbal YeahPas encore d'évaluation
- They Have The Molecular Formulas C (H O)Document33 pagesThey Have The Molecular Formulas C (H O)Iqbal YeahPas encore d'évaluation
- FLOCCULATIONDocument7 pagesFLOCCULATIONIqbal YeahPas encore d'évaluation
- CMT 574Document21 pagesCMT 574Iqbal YeahPas encore d'évaluation
- AminesDocument23 pagesAminesIqbal YeahPas encore d'évaluation
- Spme 510Document6 pagesSpme 510Iqbal YeahPas encore d'évaluation
- Cover PageDocument1 pageCover PageIqbal YeahPas encore d'évaluation
- Spe ChlorpyrifosDocument8 pagesSpe ChlorpyrifosIqbal YeahPas encore d'évaluation
- Spme 510Document8 pagesSpme 510Iqbal YeahPas encore d'évaluation
- HPLCDocument9 pagesHPLCIqbal YeahPas encore d'évaluation
- Aldehydes and KetonesDocument84 pagesAldehydes and KetonesIqbal YeahPas encore d'évaluation
- FameDocument8 pagesFameIqbal YeahPas encore d'évaluation
- FameDocument8 pagesFameIqbal YeahPas encore d'évaluation
- NovellDocument4 pagesNovellFerry AndrianPas encore d'évaluation
- Chems - List - Updated For 2020Document4 pagesChems - List - Updated For 2020gdubxPas encore d'évaluation
- Reactions of Alkenes and Alkynes Study GuideDocument17 pagesReactions of Alkenes and Alkynes Study GuideMelissa GarciaPas encore d'évaluation
- Arginine 1,4,23 PDFDocument35 pagesArginine 1,4,23 PDFLAURAPas encore d'évaluation
- PRODUCT LIST (PROTECH TELELINK) .XlsxuDocument9 pagesPRODUCT LIST (PROTECH TELELINK) .XlsxuYoussef Kaid100% (1)
- AntidotesDocument3 pagesAntidotesAbdul SalamPas encore d'évaluation
- Chemistry H3 Notes The Synthesis of ParacetamolDocument15 pagesChemistry H3 Notes The Synthesis of ParacetamolSimon KohPas encore d'évaluation
- 2015 Hrinj DrugsDocument303 pages2015 Hrinj Drugsprahul2588100% (1)
- Daftar Obat Aman Dan Berbahaya Untuk Ibu Hamil DanDocument6 pagesDaftar Obat Aman Dan Berbahaya Untuk Ibu Hamil Danirma suwandi sadikinPas encore d'évaluation
- Organic Chemistry.2nd Year. (All)Document15 pagesOrganic Chemistry.2nd Year. (All)shaiksameer7302Pas encore d'évaluation
- Rekapitulasi Tagihan Detail PortraitDocument8 pagesRekapitulasi Tagihan Detail Portraitanon_495464522Pas encore d'évaluation
- List of Drugs and Medicines Inventory REGULAR MEDICINESDocument20 pagesList of Drugs and Medicines Inventory REGULAR MEDICINESAlex SibalPas encore d'évaluation
- Dr. Laurie S. Starkey, Organic Chemistry LaboratoryDocument1 pageDr. Laurie S. Starkey, Organic Chemistry LaboratoryDavid Galiano LatorrePas encore d'évaluation
- CempakaDocument3 pagesCempakaMangos MudaPas encore d'évaluation
- Nutrient Depletion GuideDocument2 pagesNutrient Depletion GuideMichael CabarlesPas encore d'évaluation
- Harga Beli Mersifarma Ekatalog 2019Document3 pagesHarga Beli Mersifarma Ekatalog 2019Jemmy GeraldzPas encore d'évaluation
- Data P3KDocument3 pagesData P3KRsf NathanPas encore d'évaluation
- Data Pasien TB JuliDocument42 pagesData Pasien TB JuliTsubbatun NajahPas encore d'évaluation
- Chapter 1 Amine in ClassDocument72 pagesChapter 1 Amine in ClassChiu FongPas encore d'évaluation
- 13-C NMR Chemical Shift TableDocument1 page13-C NMR Chemical Shift TableSherwin John NavarroPas encore d'évaluation
- Examen 1Document12 pagesExamen 1Sahimara de JesúsPas encore d'évaluation
- CIPAC - Numbers of A.IDocument30 pagesCIPAC - Numbers of A.IAmera MasuodPas encore d'évaluation
- Data Resep Obat 02-09-2021Document633 pagesData Resep Obat 02-09-2021FARMASI CENDANAPas encore d'évaluation
- Carboxylic Acids and Carboxylic Acid Derivatives: Himilo University Course Name: Organic ChemistryDocument37 pagesCarboxylic Acids and Carboxylic Acid Derivatives: Himilo University Course Name: Organic ChemistryAbdulkarim FagaasePas encore d'évaluation
- EstersDocument5 pagesEstersNevin BakrPas encore d'évaluation
- Bioransformation2 180518095843 PDFDocument79 pagesBioransformation2 180518095843 PDFSujyoti ShakyaPas encore d'évaluation
- Daftar Obat Prak 2Document3 pagesDaftar Obat Prak 2Riza AsariPas encore d'évaluation
- Common ICU DripsDocument1 pageCommon ICU DripsSunshine Willis100% (2)
- Hey 4-N/i'ethylenedio Yphenyl Sopropyl E.: 4-MethylenedioxyphenylisopropylamineDocument2 pagesHey 4-N/i'ethylenedio Yphenyl Sopropyl E.: 4-MethylenedioxyphenylisopropylamineAnonymous FigYuONxuuPas encore d'évaluation
- 4 HeterocyclicDocument20 pages4 HeterocyclicRajesh Kumar RapoluPas encore d'évaluation