Académique Documents
Professionnel Documents
Culture Documents
Valproic Acid
Transféré par
herzi rahmatul sCopyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Valproic Acid
Transféré par
herzi rahmatul sDroits d'auteur :
Formats disponibles
VALPROIC ACID
Valproic Acid
Valproic Acid is agent that is chemically related to free fatty acids and is used
in the treatment of generalized, partial, and absence (petit mal) seizures.
It has thewidest spectrum of activity compared to the other currently
available antiepileptic drugs.
Now available in intravenous, as well as oral, form, valproic acid can be used
for the acute treatment and chronic prophylaxis of seizures.
Also a useful agent for the treatment of bipolar affective disorders and the
prevention of migraine headaches.
Its antiepileptic effect is thought to result from
its ability to increase concentrations of the neuroinhibitor -aminobutyric acid (GABA),
to potentiate the postsynaptic response to GABA,
to exert a direct effect on cellular membranes.
Bauer, Larry A,2008,hal 563
THERAPEUTIC AND TOXIC
CONCENTRATIONS
The generally accepted therapeutic range for total valproic
acid Css : 50100 g/mL.
some clinicians suggest drug concentrations as high as 175
g/mL with appropriate monitoring of serum concentrations
and possible adverse effects.
highly protein bound to albumin with typical values of 90
95%.
Plasma protein binding of valproic acid is saturable within the
therapeutic range, which results in less protein binding and
higher unbound fraction of drug at higher concentrations.
Bauer, Larry A,2008,hal 563
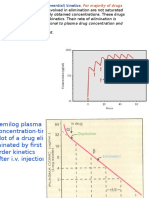
The concentration-dependent protein
binding of valproic acid causes the drug to
follow nonlinear pharmacokinetics
(fundamentally different from phenytoin)
In the case of valproic acid, when the dose
total drug steady-state concentration
less than expected, but unbound steady-
state drug concentration in a
proportional fashion.
Bauer, Larry A,2008,hal 564
unbound steady-state concentration therapeutic range for
valproic acid of 2.510.
As is the case with phenytoin, measurement of unbound
valproic acid serum concentrations should be considered in
patients with factors known to alter valproic acid plasma
protein binding.
These factors fall into three broad categories :
lack of binding protein where there are insufficient plasma
concentrations of albumin
displacement of valproic acid from albumin binding sites by
endogenous compounds
displacement of valproic acid from albumin binding sites by
exogenous compounds
Bauer, Larry A,2008,hal 565
Bauer, Larry A,2008,hal 565
Bauer, Larry A,2008,hal 565
Disease States and Conditions
that Alter Valproic Acid Plasma
Protein Binding
Bauer, Larry A,2008,hal 566
In the upper end of the therapeutic range (>75
g/mL) some patients will begin to experience the
concentration-dependent adverse effects of
valproic acid therapy : ataxia, sedation, lethargy,
and tiredness.
Other concentration-related side effects of
valproic acid therapy include:
tremor : concentrations >100 g/mL
stupor or coma : concentrations >175 g/mL
Bauer, Larry A,2008,hal 567
CLINICAL MONITORING
PARAMETERS
The goal of therapy with anticonvulsants to reduce seizure
frequency and maximize quality of life with a minimum of
adverse drug effects.
Patients should be monitored for concentration-related side
effects (local irritation of gastric mucosa)
Valproic acid serum concentrations should be measured in most
patients.
Valproic acid serum concentrations are also valuable tools to
avoid adverse drug effects. Patients are more likely to accept
drug therapy if adverse reactions are held to the absolute
minimum.
Bauer, Larry A,2008,hal 567
BASIC CLINICAL
PHARMACOKINETIC
PARAMETERS
Valproic acid is primarily eliminated by hepatic metabolism
(>95%).
Hepatic metabolism is via glucuronidation, -oxidation, and -
hydroxylation.
Over 10 metabolites have been identified for valproic acid, and
the 4-en-valproic acid metabolite may be associated with the
drugs propensity to cause hepatotoxicity.
About 15% of a valproic acid dose is recovered in the urine as
unchanged drug.
Valproic acid is eliminated almost completely by hepatic
metabolism, and it is a low hepatic extraction ratio drug.
Bauer, Larry A,2008,hal 568
This is the reason total steady-state concentrations
increase disproportionately after a valproic acid dosage
increase.
F : valproic acid bioavailability
D : valproic acid dose
: the dosage interval
ClH : hepatic clearance
However, since unbound steady-state concentrations
are only influenced by intrinsic clearance, unbound
concentrations increase in a proportional amount to
dose
Bauer, Larry A,2008,hal 568
Valproic acid volume of distribution (V = 0.15 0.2
L/kg) is also affected by
concentration-dependent plasma protein binding
and is determined by the physiologic volume of
blood (VB) and tissues (VT)
the unbound fraction of drug in the blood (fB) and
tissues (fT)
As valproic acid concentrations, unbound fraction of
drug in the bloodwhich causes an in the volume of
distribution for the drug
Bauer, Larry A,2008,hal 568
t1/2 is related to clearance and volume of distribution using
the same equation as for linear pharmacokinetics
On average, valproic acid half-life is 1218 hours in adult
patients with total concentrations within the therapeutic
range.
Valproic acid is available as three different entities (all of
them are prescribed as valproic acid equivalents)
valproic acid
sodium valproate (the sodium salt of valproic acid)
divalproex sodium (a stable coordination compound
consisting of a 1:1 ratio of valproic acid and sodium
valproate)
Bauer, Larry A,2008,hal 568
EFFECTS OF DISEASE STATES AND
CONDITIONS
ForON PHARMACOKINETICS
valproic acid, oral clearance : AND DOSING
(Cl/F) is 712 mL/h/kg and half-life is 1218 hours for adults
In children 612 years old, oral clearance and half-life equal 1020
mL/h/kg and 68 hours
Clearance rates can be higher and half-lives shorter in patients
receiving other hepatic drugmetabolizing enzyme inducers
For adults receiving other antiepileptic drugs that are enzyme
inducers, valproic acid clearance
adults is 1518 mL/h/kg and half-lives range from 4 to 12 hours
Children is 2030 mL/h/kg and half-life is 46 h
Valproic acid volume of distribution (V/F) is 0.150.2 L/kg
Bauer, Larry A,2008,hal 569
Bauer, Larry A,2008,hal570
DRUG INTERACTIONS
Valproic acid is a potent inhibitor of hepatic drug metabolizing
enzyme systems and glucuronidation
antiepileptic drugs that have their clearance rates and Css
by valproic acid-related enzyme inhibition (clonazepam,
carbamazepine, phenytoin, primidone, lamotrigine, and
ethosuximide)
Valproic acid therapy also the clearance and Css of other
drugs (zidovudine, amitriptyline, and nortriptyline)
Phenytoin, lamotrigine, rifampin, and carbamazepine can
valproic acid clr and valproic acid steady-state serum
concentrations
Cimetidine, chlorpromazine, and felbamate are examples of
Bauer, Larry A,2008,hal571
drugs that valproic acid clearance and valproic acid steady-
Aspirin, warfarin, and phenytoin all have
plasma protein binding drug interactions
with valproic acid, and these drugs have
higher unbound fractions when given
concurrently with valproic acid.
The drug interaction between valproic acid
and phenytoin deserves special examination
because of its complexity and because these
two agents are regularly used together for
the treatment of seizures
Bauer, Larry A,2008,hal571
INITIAL DOSAGE
DETERMINATION
METHODS
Pharmacokinetic dosing method
CLEARANCE ESTIMATE
VOLUME OF DISTRIBUTION ESTIMATE
HALF-LIFE AND ELIMINATION RATE CONSTANT ESTIMATE
SELECTION OF APPROPRIATE PHARMACOKINETIC MODEL AND
EQUATIONS
Literature-Based Recommended Dosing
USE OF VALPROIC ACID SERUM CONCENTRATIONS TO ALTER DOSES
Pseudolinear Pharmacokinetics Method
Pharmacokinetic Parameter Method
BAYESIAN PHARMACOKINETIC COMPUTER PROGRAMS
Bauer, Larry A,2008,hal573
Vous aimerez peut-être aussi
- Complementary and Alternative Medical Lab Testing Part 6: Liver and GallbladderD'EverandComplementary and Alternative Medical Lab Testing Part 6: Liver and GallbladderPas encore d'évaluation
- Valproic AcidDocument15 pagesValproic AcidNadya PrafitaPas encore d'évaluation
- Carboxylic Acid DerivativesDocument3 pagesCarboxylic Acid Derivativesshai padillaPas encore d'évaluation
- Anticonvulsant: Class A Group 6Document37 pagesAnticonvulsant: Class A Group 6Syahril TaminPas encore d'évaluation
- Monitoring Unbound Valproic Acid Concentration in Patients With Hypoalbuminemia 2019 PDFDocument20 pagesMonitoring Unbound Valproic Acid Concentration in Patients With Hypoalbuminemia 2019 PDFCESAR AUGUSTO CARVAJAL RENDONPas encore d'évaluation
- 10-Valproic AcidDocument49 pages10-Valproic AcidAli AmerPas encore d'évaluation
- Pharmacology of CNS: Prajogo Wibowo Faculty of Medicine Hang Tuah UniversityDocument37 pagesPharmacology of CNS: Prajogo Wibowo Faculty of Medicine Hang Tuah UniversityIda Bagus Putu SwabawaPas encore d'évaluation
- Julia Leung Depakote For The Treatment of Behavioral and Psychological Symptoms of Dementia BPSD in Geriatric Populations Work SampleDocument7 pagesJulia Leung Depakote For The Treatment of Behavioral and Psychological Symptoms of Dementia BPSD in Geriatric Populations Work Sampleapi-535645606Pas encore d'évaluation
- A Theoretical Method For Normalizing Total Serum Valproic Acid Concentration in Hypoalbuminemic PatientsDocument5 pagesA Theoretical Method For Normalizing Total Serum Valproic Acid Concentration in Hypoalbuminemic Patientsmarc daouPas encore d'évaluation
- Drug Interactions With Antiretrovirals & WarfarinDocument18 pagesDrug Interactions With Antiretrovirals & WarfarinkachapaxxPas encore d'évaluation
- Ramipril ProspectDocument18 pagesRamipril ProspectStefan Codrin CriclevitzPas encore d'évaluation
- Valproate Uses, Dosing & Side EffectsDocument15 pagesValproate Uses, Dosing & Side EffectsdrdeucePas encore d'évaluation
- Albumin: Pathophysiologic Basis of Its Role in The Treatment of Cirrhosis and Its ComplicationsDocument26 pagesAlbumin: Pathophysiologic Basis of Its Role in The Treatment of Cirrhosis and Its Complicationsravi rajPas encore d'évaluation
- DRUG SODIUM VALPROATE (Depakote, Epilim, Episenta)Document5 pagesDRUG SODIUM VALPROATE (Depakote, Epilim, Episenta)Pearl Princess Guerrero100% (2)
- Antikonvulsan OkeDocument77 pagesAntikonvulsan OkeherliaPas encore d'évaluation
- The Kidney: Organ for Drug Elimination and ClearanceDocument42 pagesThe Kidney: Organ for Drug Elimination and ClearanceSelena MoonPas encore d'évaluation
- Population Pharmacokinetics of Valproic Acid in Patients With Mania: Implication For Individualized Dosing RegimensDocument11 pagesPopulation Pharmacokinetics of Valproic Acid in Patients With Mania: Implication For Individualized Dosing RegimensKelletCadilloBarruetoPas encore d'évaluation
- Febuxostat (Uloric), A New Treatment Option For Gout: Carmela Avena-Woods Olga Hilas Author Information Go ToDocument9 pagesFebuxostat (Uloric), A New Treatment Option For Gout: Carmela Avena-Woods Olga Hilas Author Information Go ToAnadi GuptaPas encore d'évaluation
- Drug Use in Special Conditions - CLD and CKDDocument93 pagesDrug Use in Special Conditions - CLD and CKDadamu mohammadPas encore d'évaluation
- Valproic Acid in Epilepsy Clinical Trial ResultsDocument6 pagesValproic Acid in Epilepsy Clinical Trial ResultsadityaPas encore d'évaluation
- Pharmacokinetics of Anti-Epileptic DrugsDocument12 pagesPharmacokinetics of Anti-Epileptic Drugsrozina mulatPas encore d'évaluation
- HW3 PharmacologyDocument8 pagesHW3 PharmacologyMICHAEL GABRIEL JIMENEZPas encore d'évaluation
- Antiviral TherapyDocument19 pagesAntiviral TherapyMalueth AnguiPas encore d'évaluation
- Bioavailabilitas Dan Interaksi ObatDocument49 pagesBioavailabilitas Dan Interaksi ObatMovie Scene BankPas encore d'évaluation
- Excretion of Drugs: Dr. G.SAILAJA, M.Pharm - PH.D., Assoc. Professor, Dept. of PharmaceuticsDocument43 pagesExcretion of Drugs: Dr. G.SAILAJA, M.Pharm - PH.D., Assoc. Professor, Dept. of PharmaceuticsSailaja Reddy GunnamPas encore d'évaluation
- Updates on Diagnosing and Treating Acetaminophen ToxicityDocument15 pagesUpdates on Diagnosing and Treating Acetaminophen ToxicityAnumolu GoparajuPas encore d'évaluation
- Protein Binding: Naila Abbasi Assistant Professor Department of PharmacyDocument50 pagesProtein Binding: Naila Abbasi Assistant Professor Department of PharmacyMuhammad MursaleenPas encore d'évaluation
- Antidyslipidemic Drugs (Geppetti)Document32 pagesAntidyslipidemic Drugs (Geppetti)Ariel OlshevskyPas encore d'évaluation
- Levetiracetam Therapy for Seizures: Mechanism, Uses, MonitoringDocument5 pagesLevetiracetam Therapy for Seizures: Mechanism, Uses, MonitoringChrisPas encore d'évaluation
- Case Study DDD Warfarin ANSWERSDocument4 pagesCase Study DDD Warfarin ANSWERSLeyla MajundaPas encore d'évaluation
- Drug Prescription in CKD and DialysisDocument24 pagesDrug Prescription in CKD and DialysisAnitha SPas encore d'évaluation
- 11-Clarke - Pharmacokinetics and TDMDocument54 pages11-Clarke - Pharmacokinetics and TDMMiski AghniaPas encore d'évaluation
- Effexor Package InsertDocument53 pagesEffexor Package InsertpatgarettPas encore d'évaluation
- Lec - No.12 (Carbamazipine)Document13 pagesLec - No.12 (Carbamazipine)phd0780Pas encore d'évaluation
- Clinical Pharmacokinetics 2013Document68 pagesClinical Pharmacokinetics 2013Law YouPas encore d'évaluation
- Understanding Anti-Müllerian Hormone (AMH) LevelsDocument47 pagesUnderstanding Anti-Müllerian Hormone (AMH) LevelsJerry TjoanatanPas encore d'évaluation
- L2 PK Drug Drug InteractionDocument44 pagesL2 PK Drug Drug Interactionsoso22ahmed98Pas encore d'évaluation
- Lamivudine: in Vitro Studies Indicate That Zidovudine-Resistant Virus Isolates Can Become ZidovudineDocument7 pagesLamivudine: in Vitro Studies Indicate That Zidovudine-Resistant Virus Isolates Can Become ZidovudineKirtikrushna Suresh Prasad OjhaPas encore d'évaluation
- Altered Pharmacokinetics in Liver DiseasesDocument30 pagesAltered Pharmacokinetics in Liver DiseasesNailaAns100% (1)
- Auspar Apixaban 130621 PiDocument37 pagesAuspar Apixaban 130621 PiTran Minh NgocPas encore d'évaluation
- Total Proteins & Albumin AnalysisDocument16 pagesTotal Proteins & Albumin AnalysisMustafa KhandgawiPas encore d'évaluation
- Paracetamol Poisoning: G. Rajapandi 521625111 Final MbbsDocument18 pagesParacetamol Poisoning: G. Rajapandi 521625111 Final MbbsAntony PrakashPas encore d'évaluation
- Keto LogDocument7 pagesKeto LogKim Justin InfantadoPas encore d'évaluation
- GI DrugsDocument19 pagesGI DrugsHarshwardhan SinhPas encore d'évaluation
- Ivivc: in Vitro-In Vivo CorrelationDocument46 pagesIvivc: in Vitro-In Vivo CorrelationMubammad Mursaleen100% (1)
- Hepatic Encephalopathy 2Document45 pagesHepatic Encephalopathy 2Vasanth KrishnanPas encore d'évaluation
- CARMABAZEPINE (Bauer)Document5 pagesCARMABAZEPINE (Bauer)Ela chawPas encore d'évaluation
- PPT RanolazineDocument20 pagesPPT RanolazineashPas encore d'évaluation
- Food-Drug-Nutrient Interactions OverviewDocument8 pagesFood-Drug-Nutrient Interactions OverviewLorinda SorensenPas encore d'évaluation
- ValdoxanDocument27 pagesValdoxanPetre FallPas encore d'évaluation
- Summary of Product Characteristics, Labelling and Package LeafletDocument19 pagesSummary of Product Characteristics, Labelling and Package Leafletعلوبا الرايقPas encore d'évaluation
- In Vitro, Sucralfate Adsorbs Bile SaltsDocument5 pagesIn Vitro, Sucralfate Adsorbs Bile SaltsZarbakht AliPas encore d'évaluation
- AllopurinolDocument31 pagesAllopurinolbeewolPas encore d'évaluation
- PK Variability in Disease StatesDocument39 pagesPK Variability in Disease StatesDeepika ImmadiPas encore d'évaluation
- Clinical Pharmacokinetics of PHENYTOINDocument69 pagesClinical Pharmacokinetics of PHENYTOINIndira ButkoonPas encore d'évaluation
- Alpha-blockers and 5-alpha-reductase inhibitors for BPH treatmentDocument10 pagesAlpha-blockers and 5-alpha-reductase inhibitors for BPH treatmentYunitia AnjaniPas encore d'évaluation
- Peptic Ulcer Disease MedsDocument9 pagesPeptic Ulcer Disease MedsjulianpaulPas encore d'évaluation
- The Pharmacogenomics of Valproic Acid: ReviewDocument6 pagesThe Pharmacogenomics of Valproic Acid: ReviewadityaPas encore d'évaluation
- A Comprehensive Book on Experimental PharmaceuticsD'EverandA Comprehensive Book on Experimental PharmaceuticsÉvaluation : 5 sur 5 étoiles5/5 (1)
- 2Document3 pages2herzi rahmatul sPas encore d'évaluation
- PHARMACY PRESCRIPTIONDocument16 pagesPHARMACY PRESCRIPTIONherzi rahmatul sPas encore d'évaluation
- 1Document9 pages1herzi rahmatul sPas encore d'évaluation
- 1Document9 pages1herzi rahmatul sPas encore d'évaluation
- Orde 0Document6 pagesOrde 0herzi rahmatul sPas encore d'évaluation
- PHARMACY PRESCRIPTIONDocument16 pagesPHARMACY PRESCRIPTIONherzi rahmatul sPas encore d'évaluation
- Lidoka inDocument17 pagesLidoka inherzi rahmatul sPas encore d'évaluation
- HEMODYALISISDocument8 pagesHEMODYALISISherzi rahmatul sPas encore d'évaluation
- AparatusDocument15 pagesAparatusherzi rahmatul sPas encore d'évaluation
- Directly Proportional To Plasma Drug Concentration and Their Clearance Remains ConstantDocument6 pagesDirectly Proportional To Plasma Drug Concentration and Their Clearance Remains Constantherzi rahmatul sPas encore d'évaluation
- DosisDocument8 pagesDosisherzi rahmatul sPas encore d'évaluation
- Soal Tast ToelfDocument9 pagesSoal Tast Toelfherzi rahmatul sPas encore d'évaluation
- Kinetika FixDocument13 pagesKinetika Fixherzi rahmatul sPas encore d'évaluation
- Kinetika FixDocument13 pagesKinetika Fixherzi rahmatul sPas encore d'évaluation
- Contact AngleDocument33 pagesContact Angleherzi rahmatul sPas encore d'évaluation
- Soal Tast ToelfDocument9 pagesSoal Tast Toelfherzi rahmatul sPas encore d'évaluation
- Drug Absorption PDFDocument117 pagesDrug Absorption PDFAndhia DhiyaPas encore d'évaluation
- 5 6116307916568921949Document15 pages5 6116307916568921949jhafar dlexoPas encore d'évaluation
- Pharmaceutical Company Marketing PlanDocument19 pagesPharmaceutical Company Marketing PlanPalo Alto Software91% (32)
- PT Bintang Semesta Farma Semarang Semarang Nama Barang Stock Harga + 15%Document23 pagesPT Bintang Semesta Farma Semarang Semarang Nama Barang Stock Harga + 15%Gigih Kenanga SariPas encore d'évaluation
- Homeopathic PharmacyDocument376 pagesHomeopathic PharmacyBlitzone100% (12)
- Current MHRA Fees - GOV - UKDocument33 pagesCurrent MHRA Fees - GOV - UKYusuff QuadriPas encore d'évaluation
- Mahesh Sodha, Soraya Dhillon - Non-Medical Prescribing-Pharmaceutical Press (2009)Document241 pagesMahesh Sodha, Soraya Dhillon - Non-Medical Prescribing-Pharmaceutical Press (2009)saman7752100% (1)
- Rencana Kebutuhan Obat Pada Fasilitas Kesehatan Tingkat Pertama (FKTP) TAHUN 2021Document17 pagesRencana Kebutuhan Obat Pada Fasilitas Kesehatan Tingkat Pertama (FKTP) TAHUN 2021Chia paewaPas encore d'évaluation
- Glaxo SmithlineDocument3 pagesGlaxo SmithlinebhuvaneshkmrsPas encore d'évaluation
- INVESTIGATIONALNEWDRUGAPPLICATION INDAaaDocument32 pagesINVESTIGATIONALNEWDRUGAPPLICATION INDAaaHan XuPas encore d'évaluation
- Prescription WritingDocument20 pagesPrescription WritingJohn Alfred Abbago Cabilan100% (1)
- March 2022 Workbook - Impurities in Drug Products and Drug Substances - A USP ApproachDocument209 pagesMarch 2022 Workbook - Impurities in Drug Products and Drug Substances - A USP ApproachirhamfauzziPas encore d'évaluation
- 2006 Drug Trend ReportDocument49 pages2006 Drug Trend ReportkmeriemPas encore d'évaluation
- Understanding pharmaceutical care planningDocument22 pagesUnderstanding pharmaceutical care planningChristinaPas encore d'évaluation
- J K DistributorsDocument28 pagesJ K Distributorspoojaotwani611Pas encore d'évaluation
- Pharmacy Delivery SOPDocument3 pagesPharmacy Delivery SOPDoc Alex50% (4)
- APAC Regulations and Approvals Expert Working Group Analysis ReportDocument54 pagesAPAC Regulations and Approvals Expert Working Group Analysis ReportAnu PariyarathPas encore d'évaluation
- STDTRT Guidelilne Essentialdruglist SA PHCDocument392 pagesSTDTRT Guidelilne Essentialdruglist SA PHCAvinash Rangarajan100% (1)
- Registration of Generic Drugs in Central America and Mexico: ReviewDocument16 pagesRegistration of Generic Drugs in Central America and Mexico: ReviewJagdish ChanderPas encore d'évaluation
- Using The Business Model Canvas To Guide Students in Building Business PlansDocument14 pagesUsing The Business Model Canvas To Guide Students in Building Business PlansarifPas encore d'évaluation
- Ethical Principles For Pharmacy TechniciansDocument6 pagesEthical Principles For Pharmacy TechniciansGilbert OfeiPas encore d'évaluation
- NABH-100 - FAQs 2013Document11 pagesNABH-100 - FAQs 2013Priyanka Tudu100% (5)
- Klinik Winda: NO Nama Barang Satuan Harga Keterangan Beli JualDocument8 pagesKlinik Winda: NO Nama Barang Satuan Harga Keterangan Beli JualJoeliadi tariganPas encore d'évaluation
- Session 1 Nijveldt Slides (Compatibility Mode) PDFDocument22 pagesSession 1 Nijveldt Slides (Compatibility Mode) PDFNaresh BabuPas encore d'évaluation
- SoDocument60 pagesSoAhmad SahidPas encore d'évaluation
- ANDA Guidance Content FormatDocument32 pagesANDA Guidance Content FormatAjeet SinghPas encore d'évaluation
- Nigeria Medicine Prices PDFDocument71 pagesNigeria Medicine Prices PDFsumadhurainfoPas encore d'évaluation
- Kaps GuideDocument3 pagesKaps Guideirnumambreen1550% (2)
- Aziz 2018Document12 pagesAziz 2018Mr AggarwalPas encore d'évaluation
- Comparison of Drugs in MSTGDocument2 pagesComparison of Drugs in MSTGShoaib BiradarPas encore d'évaluation