Académique Documents
Professionnel Documents
Culture Documents
Curs 3 Chimie
Transféré par
victorvulpe91Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Curs 3 Chimie
Transféré par
victorvulpe91Droits d'auteur :
Formats disponibles
|
|
|
|
.
|
\
|
|
|
.
|
\
|
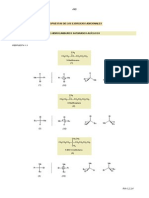
re recunoaste de procesul
chitina
celuloza
hialuronic acid
structural
glicogen depozit
glucoza imediata
energie de principala sursa
ROL
1
2
O
H
3); (n ; O) (H C C. de hidrati
dulce subst. rom sakha zaharuri
dulce glichi glucide
n 2 n
formula
grecesc
grecesc
= >
=
=
=
=
O) C ( CETONICA 2)
O) (-CH A ALDEHIDIC 1)
a carbonilic Grupare
) ( )
) ( ) 1
`]4E=] + ^`
-E=] ^+
GLUCIDE
OZE : trioze
tetroze
pentoze
hexoze
heptoze
cetotrioze
aldotrioze
cetotretoze
aldotetroze
cetopentoze
aldopentoze
cetohexoze
aldohexoze
cetoheptoze
aldoheptoze
OZIDE :
Holozide
Heterozide (heteroglucide sau glicozizi)
Oligozide
Poliozide
diozide (diglucide)
triozide (triglucide)
tetrozide (tetraglucide)
pentozide (pentaglucide)
hexozide (hexaglucide)
etc.
glicogen
amidon
celuloza
etc.
chimica Comp.
re polimeriza de Gr.
C de Nr.
a carbonilic Gr.
OZE : trioze
tetroze
pentoze
hexoze
heptoze
cetotrioze
aldotrioze
cetotretoze
aldotetroze
cetopentoze
aldopentoze
cetohexoze
aldohexoze
cetoheptoze
aldoheptoze
OZIDE :
Holozide
Heterozide (heteroglucide sau glicozizi)
Oligozide
Poliozide
diozide (diglucide)
triozide (triglucide)
tetrozide (tetraglucide)
pentozide (pentaglucide)
hexozide (hexaglucide)
etc.
glicogen
amidon
celuloza
etc.
simple zaharide
simple zaharuri
de monozahari
|
|
|
|
.
|
\
|
|
.
|
\
|
=
=
|
.
|
\
|
=
=
undare sec restul
primare 2 sau 1
) OH ( alcoolice grupari
) O C ( cetonica
) O HC ( aldehidica
a carbonilic grupare
C atomi de nr
cetononoze
aldononoze
nonoze
cetooctoze
aldooctoze
octoze
e cetoheptoz
e aldoheptoz
heptoze
cetohexoze
aldohexoze
hexoze
e cetopentoz
e aldopentoz
pentoze
e cetotetroz
e aldotetroz
tetroze
cetotrioze
aldotrioze
trioze
\
|
a carbonilic grupare
xicetone) (polihidro CETOZE
) xialdehide (polihidro ALDOZE
C
C
CH
OH
O
H
H
2
OH
gliceraldehida
C
CH
2
O
CH
2
OH
OH
dihidroxiacetona
C
C*
CH
OH
O
H
H
2
OH
gliceraldehida
D
C
C*
CH
O
H
H
2
OH
gliceraldehida
HO
-
L
-
STRUCTURA SI IZOMERIA TRIOZELOR
( )
( )
ie enantiomer
rectum R D izomer
istrum sin S L izomer
Fischer prezentare Re =
)
`
=
=
STRUCTURA SI IZOMERIA ALDOTETROZELOR
C
C*
OH
O H
H
OH
C*
H
CH
2
OH
D - eritroza
( E
1
)
C
C
O H
H
C* H
CH
OH
HO
HO
L- eritroza
(E2)
D - tetroza
(T
1
L- tetroza
(T
2
)
)
C
C*
O H
H
OH
C*
H
CH
2
OH
C
C*
OH
O H
H
C*
H
C
2
OH
HO
HO
H
2
zomeri diastereoi izomeri
T E
T E
T E
T E
T T
E E
i enantiomer de perechi 2
2 2
1 2
2 1
1 1
2 1
2 1
= =
)
`
CALCULAREA NR DE STEREOIZOMERI (x)
16 2 x 4, n
8 2 x 3, n
4 2 x 2, n
4
3
2
= = =
= = =
= = =
CALCULAREA NR DE STEREOIZOMERI (x) CALCULAREA NR DE STEREOIZOMERI (x)
|
|
.
|
\
|
=
=
=
asimetrici C de atomi de nr. n
izomeri de nr. x
n
2 x
C
C*
OH
O
H
H
OH
C*
H
C
2
OH
C*
H
C*
OH
H
HO
D - glucoza
C
C*
OH
O
H
H
C*
H
C
2
OH
C*
H
C*
OH
H
HO
HO
D - galactoza
C
C*
O H
H
OH
C* H
C
2
OH
C*
H
C*
OH
H
HO
HO
D - manoza
H
H
H
STRUCTURA SI IZOMERIA ALDOHEXOZELOR
structura
2 pozitia O C functia =
ra nomenclatu
levuloza
xiluloza
ribuloza
" uloza "
|
|
.
|
\
|
CARACTERISTICELE GENERALE ALE CETOZELOR
CALCULAREA NR DE STEREOIZOMERI (x)
|
|
.
|
\
|
=
=
=
asimetrici C de atomi de nr. n
izomeri de nr. x
n
2 x
C atom penultimul la de OH pozitia L D, ia configurat
8 2 x 3; n ; cetohexoze
4 2 x 2; n e; cetopentoz
1) (n ; 2 x izomeri nr.de
3
2
n
|
|
.
|
\
|
= = =
= = =
< =
C
O
OH
C*
H
C*
H
HO
CH
2
OH
CH
2
OH
C
O
OH
C*
OH
C*
H
H
D-xiluloza
D-ribuloza
CH
2
OH
CH
2
OH
STRUCTURA SI IZOMERIA CETOPENTOZELOR
|
|
.
|
\
|
e =
4
2
C
C
epimeri galactoza D si manoza D
galactoza D si glucoza D
ribuloza D si xiluloza D
exemple
) C (1 deosebire singura 1 izomerie EPIMERIA
REPREZENTANTI semnificatie biochimica
C
O
OH
C*
H
C*
H
HO
CH
2
OH
CH
2
OH
OH
C*
H
C
O
OH
C*
H
C*
H
HO
CH
2
OH
CH
2
OH
OH
C*
H
OH
C*
H
D-fructoza
(sau levuloza)
D-sedoheptuloza
(cetoheptoza)
ARGUMENTE
0
D
0
D
2
5 52 [ glc de veche sol.
2 112 [ glc de proaspata sol.
diferita rotatorie putere
SO cu decolorata fucsina de a recolorare
+ =
+ = |
=
2)
1)
ARGUMENTE ce contravin reprezentarii Fischer
EXPLICATIA
7 52 ] [ amestec anomerie
0
D
+ = o => | , o
|
|
.
|
\
|
+ =
+ =
7 18 ] [ glucoza D
2 112 ] [ glucoza D
izomeri 2 a existenta
0
D
0
D
ANOMERI
Izomerii
ROTATORII PUTERII SCHIMBAREA
)
`
= E MUTAROTATI
JUSTIFICAREA CHIMICA
R
aldehida
CHO R
+
OH R R
H
OH
alcool
hemiacetal
1
C O
1
Formarea de hemiacetali interni ciclici
C
H
C H
C HO
OH
CH
2
OH
OH
C
H
OH C H
H O
+H
2
O
C
H
C H
C HO
OH
CH
2
OH
OH
C
H
O H C H
H O
OH
- H
2
O
C
H
C H
C HO
OH
CH
2
OH
OH
C
H
C H
H O
O
Forma
aldehida libera
Hidrat de
aldehida
Hemiacetal
Forma glucopiranozica
(punte oxidica 1-5)
Forma liniara
H
1
2
3
4
5
6
REPREZENTARE propusa de TOLLENS
|
|
|
.
|
\
|
+
+
furanoza OH) ( aldeh.) 2)(Grup.
piranoza OH) ( aldeh.) (Grup. 1)
HOH
4 1
HOH
5 1
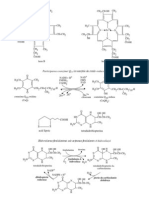
POSIBILITATI de CICLIZARE pt ALDOZE
(GLUCOZA)
O) (5atomi FURAN FURANOZA
O) (6atomi PIRAN PIRANOZA
Etimologia
e
e
CH
2
CH
CH
CH
CH
O
O
CH
CH CH
CH
Structura piranului
CH
2
PIRAN
CH
CH
CH
CH
O
CH
CH CH
CH
Structura furanului
O
FURAN
MODIFICAREA FUNCTIEI REDUCATOARE
|
|
.
|
\
|
=
acetalica pseudo
ica semiacetal
ica hemiacetal forma O) CH e( reducatoar Funct.
POSIBILITATI de CICLIZARE pt CETOZE
(FRUCTOZA)
|
.
|
\
|
|
.
|
\
|
+
+
ica hemiacetal
ica semiacetal
nica pseudoceto cetonica functia
) furanozic nucleu ) OH ( ) cetonica . gr .( 2
piranozic nucleu ) OH ( ) cetonica . gr .( 1
HOH
5 2
HOH
6 2
|
o
-
)
`
-
-
izomer
izomer
anomerie C atom acestui OH pozitia
cetoze ) 2 C ( C atom 1 aparitia
aldoze ) 1 C ( C atom 1 aparitia
CONSECINTELE CICLIZARII
e
PIRANOZICA
FURANOZICA
FORMA -
IZOMERI -
GLUCIDELE TOATE
=
oza glucofuran D
oza glucofuran D
oza glucopiran D
oza glucopiran D
amestec a) (sol.apoas Glc
)
`
piranoza
furanoza
HOWARTH
oxidica punte
liniara
TOLLENS
ANOMERIA
RE REPREZENTA
Concluzie
H-C-OH
HO-C-H
H-C-OH
H-C
CH
2
OH
O
H-C-OH
HO-C-H
H-C-OH
H-C
CH
2
OH
O
H OH
HO H
H-C-OH
HO-C-H
H-C
H-C-OH
CH
2
OH
O
H OH
H-C-OH
HO-C-H
H-C
H-C-OH
CH
2
OH
O
H HO
o-D-glucopiranoza |-D-glucopiranoza o-D-glucofuranoza |-D-glucoofuranoza
C
C
C
C
ANOMERII D-GLUCOZEI (reprezentare Tollens)
HO-C-H
H-C-OH
H-C-OH
CH
2
O
OH
CH
2
OH
HO-C-H
H-C-OH
H-C-OH
CH
2
O
OH
CH
2
OH
HO-C-H
H-C-OH
H-C
CH
2
OH
O
OH
CH
2
OH
HO-C-H
H-C-OH
H-C
CH
2
OH
O
OH
CH
2
OH
o-D-fructopiranoza |-D-fructopiranoza
o-D-fructofuranoza |-D-fructofuranoza
C
C C
C
ANOMERII D-FRUCTOZEI (reprezentare Tollens)
H-C-OH
HO-C-H
H-C-OH
H-C
CH
2
OH
O
H
OH
C
sau
CH
2
OH
C O
H
H
OH HO
H
OH
C
C C
C
H
HO
H
sau
CH
2
OH
O
H
H
OH
HO
H
OH
H
OH
H
1
2
3
4
5
6
o-D-glucopiranoza
Transformarea reprezent. Tollens in reprez. Howarth
(alfa-D-glucopiranoza)
H-C-OH
HO-C-H
H-C-OH
H-C
CH
2
OH
O
H OH
C
sau
sau
CH
2
OH
O
H
H
OH
HO
H
OH
H
OH
H
|-D-glucopiranoza
Transformarea reprezent. Tollens in reprez. Howarth
(beta-D-glucopiranoza)
HO-C-H
H-C-OH
H-C-OH
CH
2
O
CH
2
OH
OH
C
sau
O
H
H
OH
HO
H
HO
H
OH
1
2
3
4
5
6
CH
2
OH
H
o-D-fructopiranoza
Transformarea reprezent. Tollens in reprez. Howarth
(alfa-D-fructopiranoza)
HO-C-H
H-C-OH
H-C
CH
2
OH
O
OH
C
CH
2
OH
O
H OH
HO
H
H
OH
1
2
3
4
5
6
CH
2
OH
sau
HOH
2
C
o-D-fructofuranoza
Transformarea reprezent. Tollens in reprez. Howarth
(alfa-D-fructofuranoza)
HO-C-H
H-C-OH
H-C
CH
2
OH
O
OH C
CH
2
OH
sau
CH
2
OH
O
H
OH
HO
H
H
OH
1
2
3
4
5
6
HOH
2
C
|-D-fructofuranoza
Transformarea reprezent. Tollens in reprez. Howarth
(beta-D-fructofuranoza)
H-C-OH
H-C-OH
H-C
CH
2
OH
O
OH
C
H
sau
O
H OH
H
OH
H
OH
1
2 3
4
5
HOH
2
C
O
HOH
2
C
sau
H
o-D-ribofuranoza
Transformarea reprezent. Tollens in reprez. Howarth
(alfa-D-ribofuranoza)
O
O
Forma baie
Forma scaun
=
SCAUN forma
BAIE forma
IE CONFIGURAT plan hexagonal Ciclul
CONFIGURATIA BAIE-SCAUN
H
OH
HO
H
H
OH
OH
CH
2
OH
H
O
H
H
OH
HO
H
H
OH
H
CH
2
OH
H
O
OH
o - glucoza |- glucoza
CONFIGURATIA BAIE-SCAUN pt GLUCOZA
(
|
|
|
.
|
\
|
hidroxilic O un pierd care oze deoxioze
manoza D
xiloza D
galactoza D
glucoza D
de monozahari
HO - H
2
C
5
O
H
H
OH
OH
OH
H
H
1
2 3
4
HO - H
2
C
5
O
H
H
H
OH
OH
H
H
1
2 3
4
b - D - riboza b -2-deoxi - D - riboza
derivati
) acetilata (
2
NH ) 2 C ( OH inlocuirea
CH
2
OH
O
H
H
OH
HO
H
OH
H
NH
2
H
D-galactozamina
CH
2
OH
O
H
H
OH HO
H
OH
H
NH-C-CH
3
H
O
N-acetil-D-glucozamina
H - C - OH
HO - C - H
H - C - OH
H - C -OH
HO
O
C
HO O
C
Acid
D-glucuronic
derivati
COOH OH rea transforma
Definiie = lactona acidului 2,3-endiol gulonic
orbic dehidroasc L acid ascobic L acid
oxidare
}
|
|
.
|
\
|
=
=
+
orbic dehidroasc mono L acid
liber radical
redox sistem
orbic dehidroasc L acid
ascorbic L acid
BIOCHIMICE FUNCTII
Acid
L-ascorbic
O = C
H - C
HO - C - H
CH
2
OH
HO - C
HO - C
O
O = C
H - C
HO - C - H
CH
2
OH
O = C
O
Acid
dehidro-
L-ascorbic
O = C
OH
H OH
OH
OH
OH
OH
H
H
H
H
H
Mioinzitolul (Hexa-alcool derivat de la ciclohexan)
Vous aimerez peut-être aussi
- Biochimie LP4Document5 pagesBiochimie LP4Claudia TeodoraPas encore d'évaluation
- Test 3 2015Document5 pagesTest 3 2015Ioana PascaPas encore d'évaluation
- In Procesul de Fabricare A Aldehidei Acetice Prin Hidratarea AcetileneiDocument1 pageIn Procesul de Fabricare A Aldehidei Acetice Prin Hidratarea AcetileneiNicoleta EnachePas encore d'évaluation
- Test Tip ProblemeDocument4 pagesTest Tip ProblemeMaria DumitruPas encore d'évaluation
- Glucides PDFDocument6 pagesGlucides PDFolfa100% (1)
- Ciobanu, D - Probleme de Chimie RezolvateDocument216 pagesCiobanu, D - Probleme de Chimie Rezolvateeminesind100% (4)
- Valori NutritiveDocument5 pagesValori NutritiveLucian PopaPas encore d'évaluation
- Basul Toba MareDocument2 pagesBasul Toba MarePruna Andrei100% (1)
- Chimie Organica BookletDocument161 pagesChimie Organica BookletMihnea Prisecaru100% (1)
- Test Formule ChimiceDocument7 pagesTest Formule ChimiceGeorgiana Pristanda0% (1)
- Teste Grila ChimieDocument4 pagesTeste Grila ChimieMarica SirghiPas encore d'évaluation
- Chimie 8 - Chimie OrganiqueDocument14 pagesChimie 8 - Chimie OrganiqueNathan CohenPas encore d'évaluation
- Chapitre Glucides - pr.BAKRI2010Document60 pagesChapitre Glucides - pr.BAKRI2010Midouri DjafferPas encore d'évaluation
- Scimd Glu 1 2014Document78 pagesScimd Glu 1 2014AisSa KeRrøùùm0% (1)
- Lipides Scimd 2005 GastonDocument60 pagesLipides Scimd 2005 Gastonantaafall464Pas encore d'évaluation
- Poly 2012-2013Document172 pagesPoly 2012-2013anfelPas encore d'évaluation
- Du Monosaccharide Au Polysaccharide: DonnéesDocument15 pagesDu Monosaccharide Au Polysaccharide: DonnéesImanePas encore d'évaluation
- Tp2a1 2Document11 pagesTp2a1 2univers_a91% (11)
- Ejercicios Resueltos de Isomeria OpticaDocument113 pagesEjercicios Resueltos de Isomeria Opticaargentina-2009100% (2)
- Bioch1an16 GlucidesDocument163 pagesBioch1an16 GlucidesBilliez Pierre-yvesPas encore d'évaluation
- Oses Osides 2016Document43 pagesOses Osides 2016Ingenieur Agro100% (1)
- Biochimie Structurale GLUCIDESDocument17 pagesBiochimie Structurale GLUCIDESDrissPas encore d'évaluation
- Formule GlucidicDocument8 pagesFormule GlucidicAlina JskPas encore d'évaluation
- TD de Biochimie 1Document6 pagesTD de Biochimie 1Mahdjouba Benchouche100% (1)
- (Glucide-Lipide PR KABBAJDocument165 pages(Glucide-Lipide PR KABBAJskeleton93Pas encore d'évaluation
- Tables MsDocument5 pagesTables MsSimoBal-ghaouiPas encore d'évaluation
- MEtabolisme Des GlucidesDocument16 pagesMEtabolisme Des GlucidesOtmane HajiPas encore d'évaluation
- BIOCHIMIE STRUCTURALE S3 Cours GLucide-2Document78 pagesBIOCHIMIE STRUCTURALE S3 Cours GLucide-2Oumarou KontaPas encore d'évaluation
- Les Glucides PDFDocument41 pagesLes Glucides PDFLhossine AliPas encore d'évaluation
- TD GlucidesDocument12 pagesTD GlucidesAhmed LarbiPas encore d'évaluation
- TD Glucides 2018-2019Document2 pagesTD Glucides 2018-2019the world العالم0% (2)
- TD: Structure Des Glucides: Exercice 1Document6 pagesTD: Structure Des Glucides: Exercice 1MelissaPas encore d'évaluation
- 5 - Les LipidesDocument18 pages5 - Les LipidesFella BoukenaouiPas encore d'évaluation
- ALKENIDocument2 pagesALKENImikraaPas encore d'évaluation
- Biochimie Structurale - Enzymes CoenzymesDocument22 pagesBiochimie Structurale - Enzymes CoenzymeszlimitounePas encore d'évaluation
- TP D'éléctrochimieDocument7 pagesTP D'éléctrochimieHanou BouPas encore d'évaluation
- Sol Ex OptionDocument17 pagesSol Ex OptionHicham BoulahdrtPas encore d'évaluation
- Chap 5 LES GLUDocument12 pagesChap 5 LES GLUKhadim BEYEPas encore d'évaluation
- Tarea de QuimicaDocument4 pagesTarea de Quimicasamuelsalgueiro91Pas encore d'évaluation
- Chapitre Glucides - pr.BAKRI2010Document60 pagesChapitre Glucides - pr.BAKRI2010Oussama ChahirPas encore d'évaluation
- Cours de Biochimie Structurale Chap 1 Glucides-1-2-1Document40 pagesCours de Biochimie Structurale Chap 1 Glucides-1-2-1StellaPas encore d'évaluation
- Aldehydfes Et Cetones1Document6 pagesAldehydfes Et Cetones1Abdoul karim SamakePas encore d'évaluation
- Hininger Favier Isabelle P02Document17 pagesHininger Favier Isabelle P02Djallal HassaniPas encore d'évaluation
- Les GlucidesDocument11 pagesLes GlucidesSohayb BelouettarPas encore d'évaluation
- Aldehydes Ce TonesDocument31 pagesAldehydes Ce Tonesmariem outahmiditPas encore d'évaluation
- Bio Chimie 1Document7 pagesBio Chimie 15bf9nrq47pPas encore d'évaluation
- Formule VitamineDocument12 pagesFormule Vitaminealex_andra_22Pas encore d'évaluation
- Orga - 05-Chap 23Document7 pagesOrga - 05-Chap 23robinsonsamuelsonPas encore d'évaluation
- PCEM1 GlucidesDocument34 pagesPCEM1 Glucidesmonsieurweller100% (1)
- Structure Des Lipides: H C CH CH CH CH CoohDocument7 pagesStructure Des Lipides: H C CH CH CH CH CoohRodina 16Pas encore d'évaluation
- Exercices Avec Solution SV2-STU2Document18 pagesExercices Avec Solution SV2-STU2SaoudPas encore d'évaluation
- AcilovanjeDocument12 pagesAcilovanjeJankovic NatasaPas encore d'évaluation
- Tabeaux RMNDocument4 pagesTabeaux RMNYc Yacine100% (1)
- CholestérolDocument20 pagesCholestérolDes PamelaPas encore d'évaluation
- GlucidesDocument13 pagesGlucidesTimi BelaidiPas encore d'évaluation
- Corrigés Leçon 6 - Composés Oreganiques OxygénésDocument5 pagesCorrigés Leçon 6 - Composés Oreganiques Oxygénésholyeric50Pas encore d'évaluation
- 4 - Les GlucidesDocument27 pages4 - Les GlucidesFella BoukenaouiPas encore d'évaluation
- Chap6 Acidobasique2021Document3 pagesChap6 Acidobasique2021ateich simohamedPas encore d'évaluation
- Les GlucidesDocument47 pagesLes Glucideszouaouisomia13Pas encore d'évaluation
- Aldehydes Et CetonesDocument24 pagesAldehydes Et CetonesAhmed Ben AmmarPas encore d'évaluation